Recombined long-acting glucagons peptide analogue and preparation method thereof
一种胰高血糖素、类似物的技术,应用在重组长效胰高血糖素样肽类似物及其制备领域,能够解决长半衰期、强生物活性等问题,达到延长半衰期、提高疗效、蛋白表达效率高的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The construction of embodiment 1 genetically engineered bacteria
[0045] The exendin-4-HSA gene sequence was synthesized according to SEQ ID NO: 3, and the yeast codon was optimized. The methanolic yeast KEX2 restriction sequence KR and the xho1 restriction site were introduced into the N-terminal of the exendin-4 sequence, and the Not1 site was introduced into the C-terminal of the HAS. The synthesized fragment was double-digested with XhoI and NotI, and the vector pPICZαA plasmid was treated with XhoI and NotI double-digested at the same time, the fragments were recovered respectively, ligated and transformed into DH5α, and pPICZα-exendin-4-HSA transformants were screened. The correct pPICZα-exendin-4-HSA identified by sequencing was digested with sacI, electroporated to methanolic yeast X33, screened for zeocin resistance on YPD plate, and positive transformants were obtained. Pick a single colony, place it in a 250ml shake flask containing 25ml BMGY medium, and cu...
Embodiment 2
[0046] The cultivation and expression of embodiment 2 genetically engineered bacteria
[0047] Shake flask strain culture: 5 Erlenmeyer flasks with a capacity of 1L, each containing 200ml of YPD medium, the medium formula is 10g of yeast extract (1%), 20g of peptone (2%), and 20g of glucose (2%). Dissolve in deionized water and make up to 1L, steam sterilize at 115°C for 20 minutes. Each bottle was connected with 100 ul of the glycerol strain obtained in Example 1, and cultured on a shaker at 30° C. at 300 rpm for 24 hours. At this time, the bacterial concentration OD600 was between 6-20.
[0048] 30L tank fermentation culture: 30L automatic fermentation tank (Biostat c-dcu, Sartourius), specific operation can refer to its instruction manual. 15L of basal salt medium is filled in a 30L tank, and the formula is CaSO 4 2H 2 O 0.93g / l, K 2 SO 4 18.2g / l, MgSO 4 7H2O, KOH 4.13g / l, H 3 PO 4 26.7ml / l, 40g / l glycerin were dissolved in deionized water and adjusted to 15L. Co...
Embodiment 3
[0049] Example 3 Separation and Purification of Genetically Engineered Bacteria Expression Products
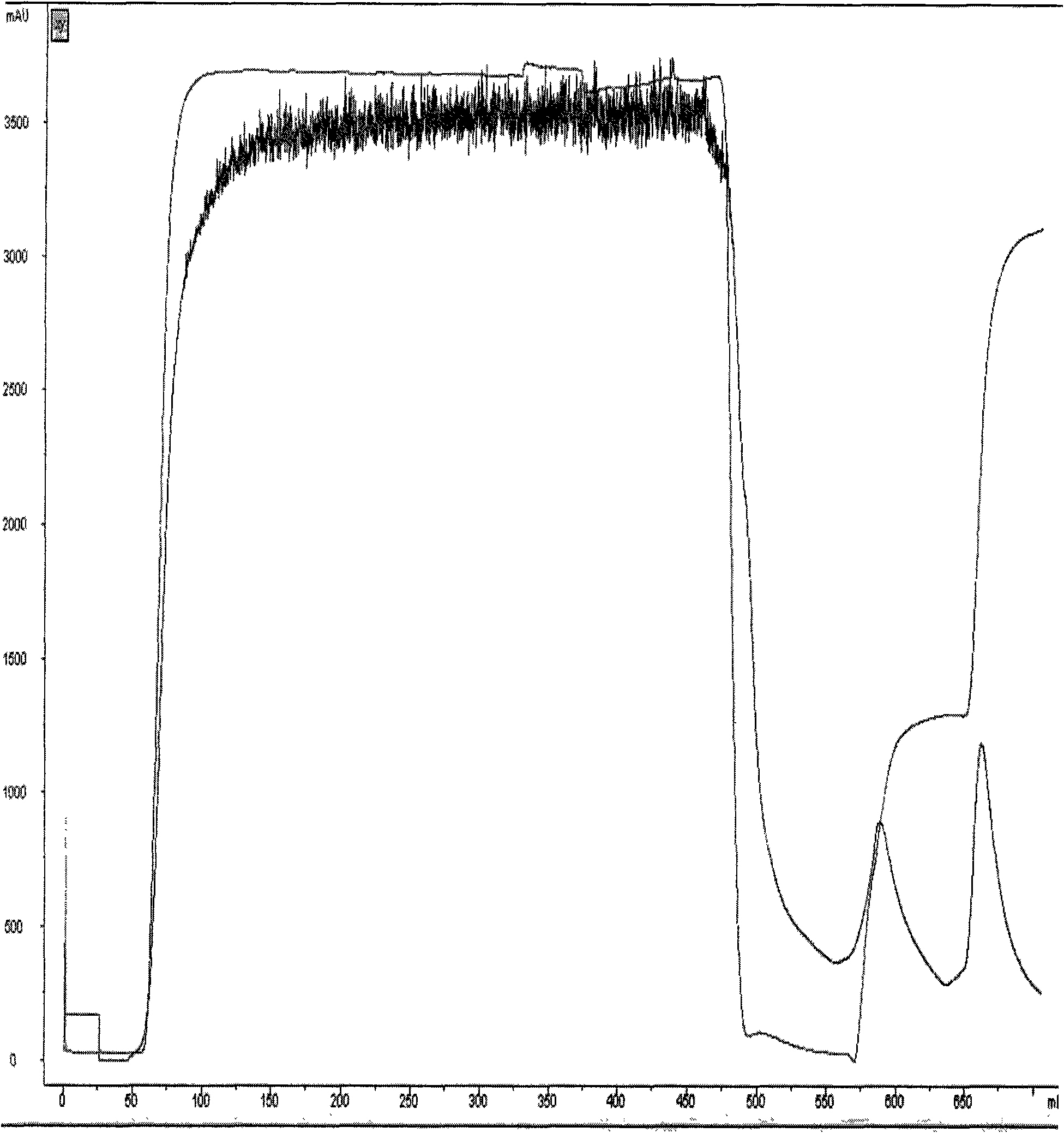
[0050] Blue dye affinity chromatography: adjust the pH of the fermentation supernatant obtained in Example 2 to 7.0 with 6M NaOH, and load it into blue dye affinity chromatography equilibrated with 20 mM phosphate at pH 7.0 and 500 mM NaCl buffer The column has a column bed diameter of 100 mm, a column bed height of 15 cm, and a column bed volume of 1000 ml. The flow rate is 8L / hour, and the sample is loaded after 3 hours. After loading the sample, first wash off unbound substances with 20 mM phosphate buffer at pH 7.0 and 128 mM KSCN buffer, and then wash off the fusion protein bound on the column with 20 mM phosphate buffer at pH 7.0 and 230 mM KSCN buffer. The protein peak was collected for detection by ultraviolet absorption at 280nM, and a total of 3200ml of the eluate was collected. Blue dye affinity chromatogram such as figure 2 shown
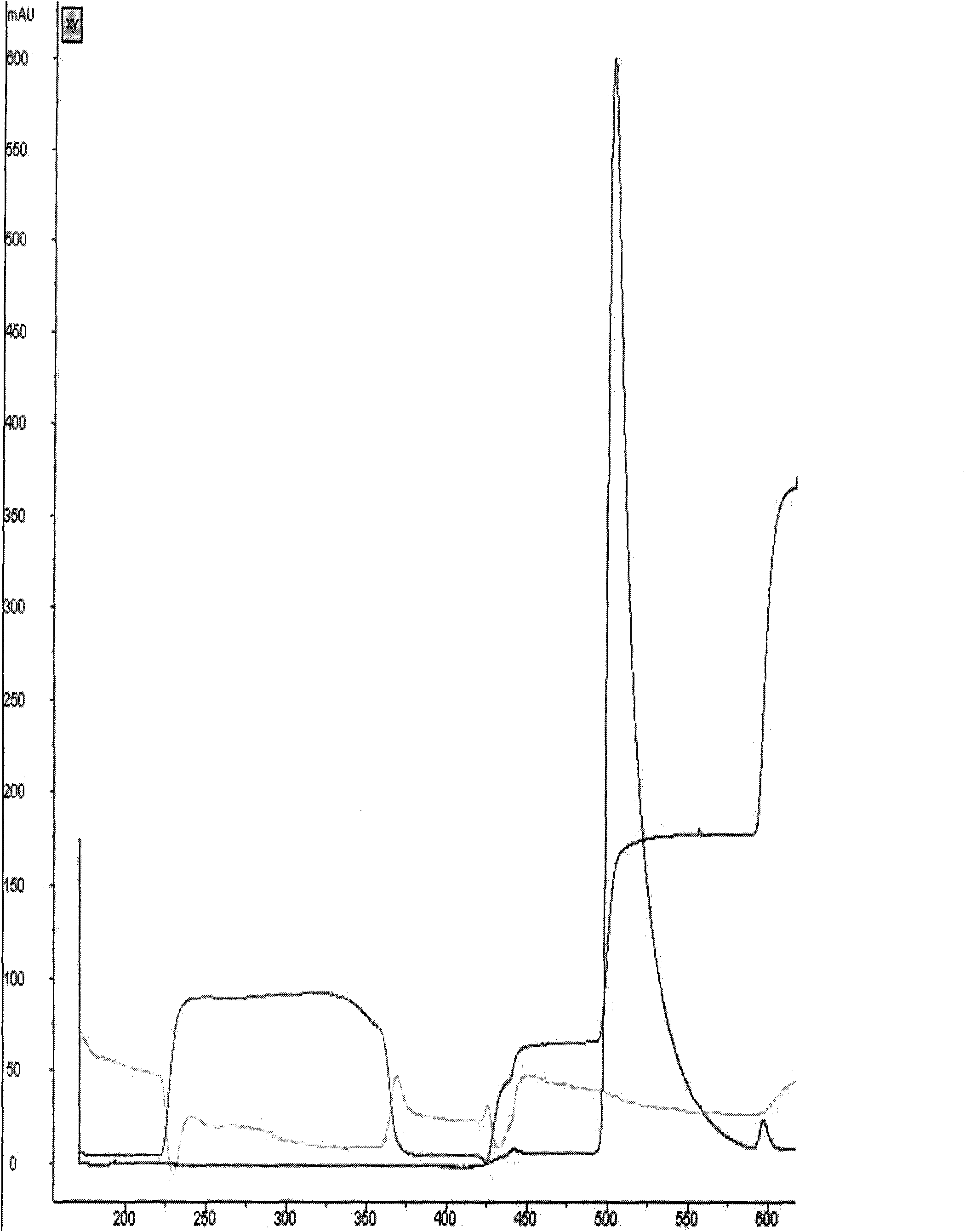
[0051] Hydrophobic chromatog...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com