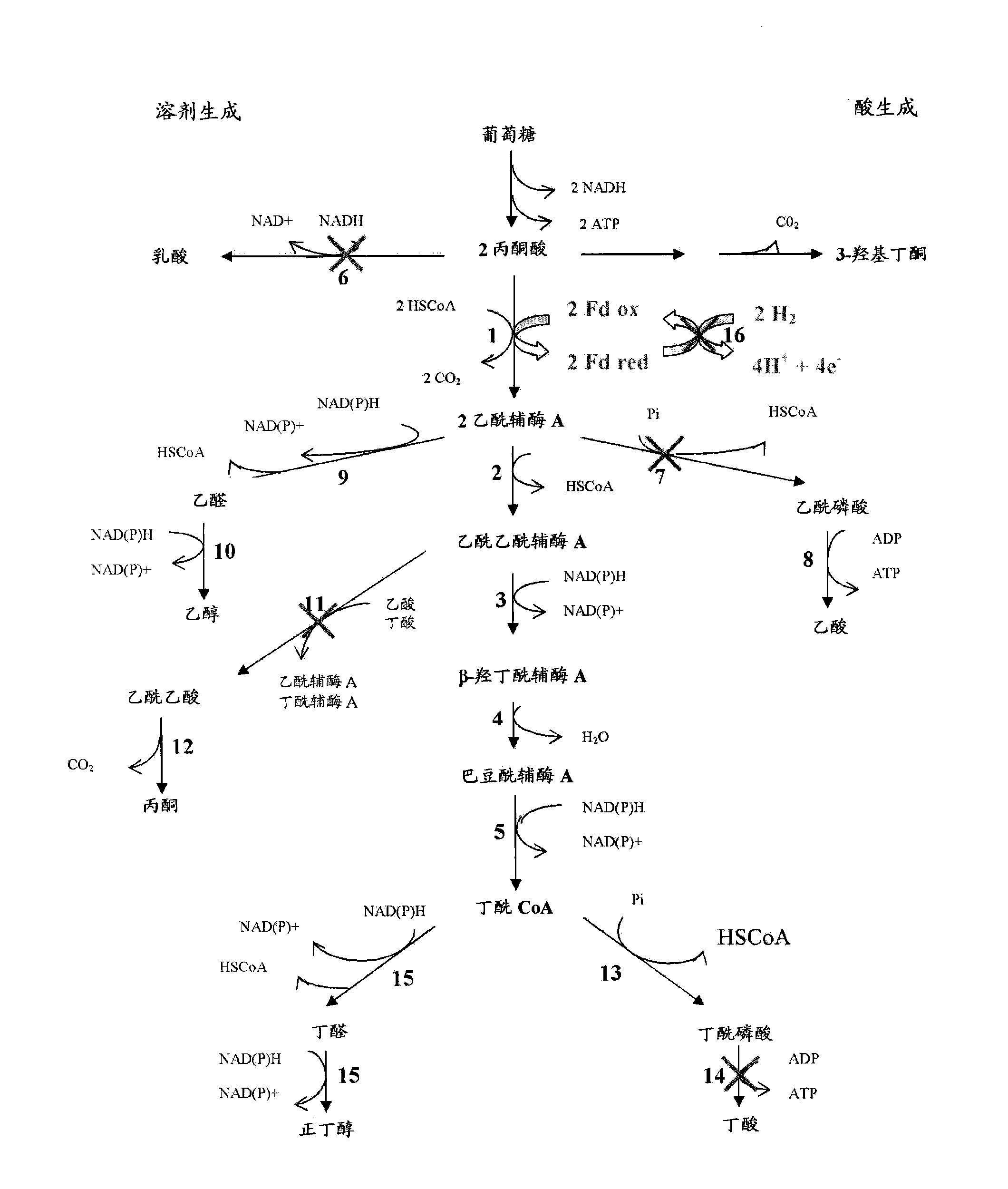
Process for the biological production of n-butanol with high yield
一种正丁醇、微生物的技术,应用在以高产率生物产生正丁醇领域,能够解决连续培养不稳定性等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Construction of a butyrate-incapable strain: Clostridium acetobutylicum Δcac1515ΔuppΔbuk
[0051] To delete the buk gene, the homologous recombination strategy described by Croux & Soucaille (2006) in patent application PCT / EP2006 / 066997 was used. This strategy allows the insertion of an erythromycin resistance cassette while deleting most of the associated genes. The buk deletion cassette in pCons::upp was constructed as follows.
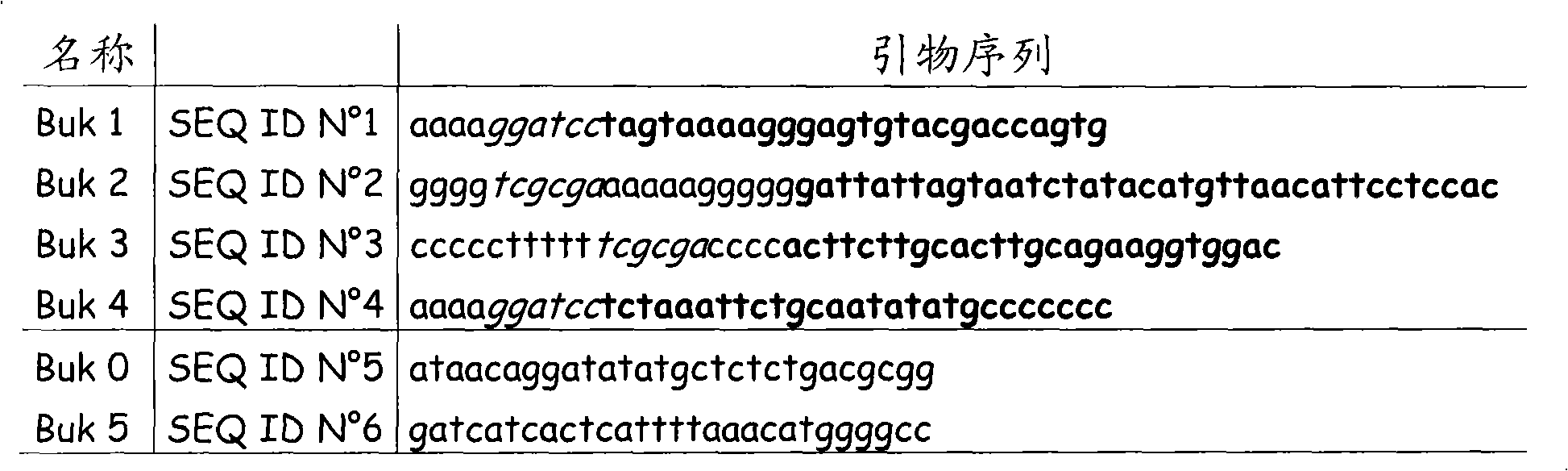
[0052] Table 1: Primer sequences
[0053]
[0054] Two DNA fragments surrounding Buk were PCR amplified with Pwo polymerase using total DNA from Clostridium acetobutylicum as template and two pairs of specific oligonucleotides. Two DNA fragments were obtained using primer pairs BUK 1-BUK2 and BUK 3-BUK 4, respectively. Primers BUK 1 and BUK 4 both introduce a BamHI site, while primers BUK 2 and BUK 3 have a complementary region that introduces a NruI site. The DNA fragments BUK 1-BUK 2 and BUK 3-BUK 4 were combined in a PCR fusion exp...
Embodiment 2
[0058] Construction of a strain incapable of producing butyrate and acetone: Clostridium acetobutylicum Δcac1515 Δupp ΔbukΔctfAB
[0059] To delete the ctfAB gene, the homologous recombination strategy described by Croux & Soucaille (2006) in patent application PCT / EP2006 / 066997 was used. This strategy allows the insertion of an erythromycin resistance cassette while deleting most of the associated genes. The ctfAB deletion cassette in pCons::upp was constructed as follows.
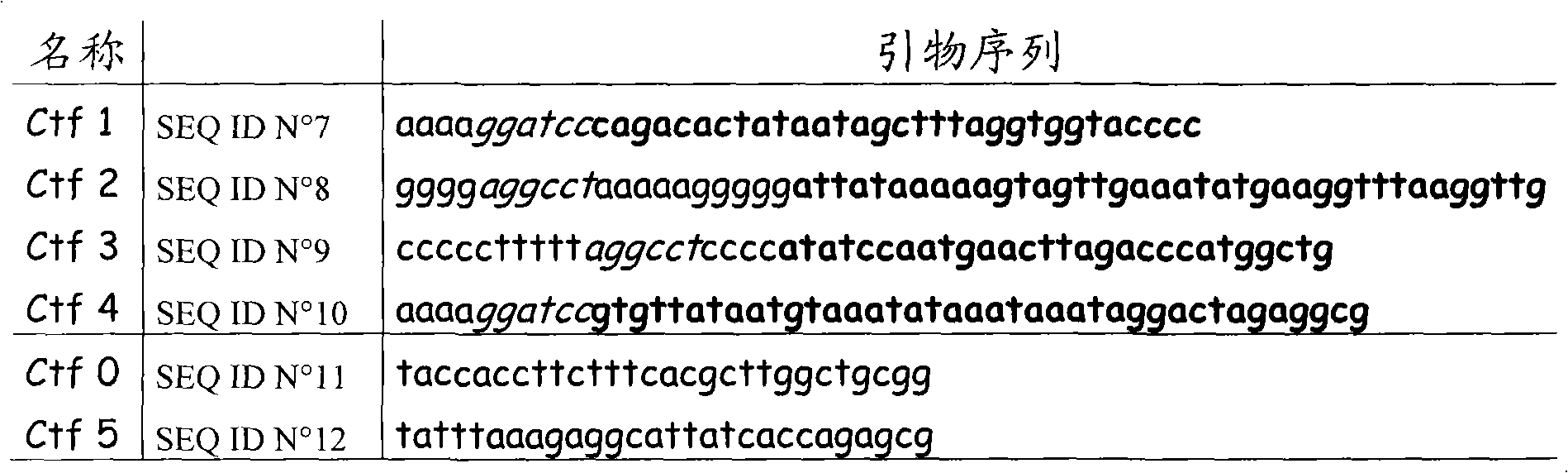
[0060] Table 2: Primer sequences
[0061]
[0062]Two DNA fragments surrounding ctfAB were PCR amplified with Pwo polymerase using total DNA from C. acetobutylicum as template and two pairs of specific oligonucleotides. Two DNA fragments were obtained using primer pairs CTF 1-CTF2 and CTF 3-CTF 4, respectively. Primers CTF 1 and CTF 4 both introduce a BamHI site, while primers CTF 2 and CTF 3 have complementary regions that introduce a StuI site. DNA fragments CTF 1-CTF 2 and CTF 3-CTF 4 were combi...
Embodiment 3
[0066] Construction of a strain incapable of producing butyrate, acetone and acetate: Clostridium acetobutylicum Δcac1515ΔuppΔbukΔctfABΔldh
[0067] To delete the ldh gene, the homologous recombination strategy described by Croux & Soucaille (2006) in patent application PCT / EP2006 / 066997 was used. This strategy allows the insertion of an erythromycin resistance cassette while deleting most of the associated genes. The ldh deletion cassette in pCons::upp was constructed as follows.
[0068] Table 3: Primer sequences
[0069]
[0070] Two DNA fragments surrounding ldh (CAC267) were PCR amplified with Pwo polymerase using total DNA from Clostridium acetobutylicum as template and two pairs of specific oligonucleotides. Using primer pairs LDH 1-LDH 2 and LDH 3-LDH 4, DNA fragments of 1135bp and 1177bp were obtained, respectively. Primers LDH 1 and LDH 4 both introduce a BamHI site, while primers LDH 2 and LDH 3 have complementary regions that introduce a StuI site. DNA fragm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com