Oxidation resisting dipeptide medicament intercalated houghite and preparation method thereof
A technology for oxidizing dipeptide and hydrotalcite, which is applied in the direction of pharmaceutical formulations, drug combinations, dipeptide components, etc., to achieve good thermal stability and shorten crystallization time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
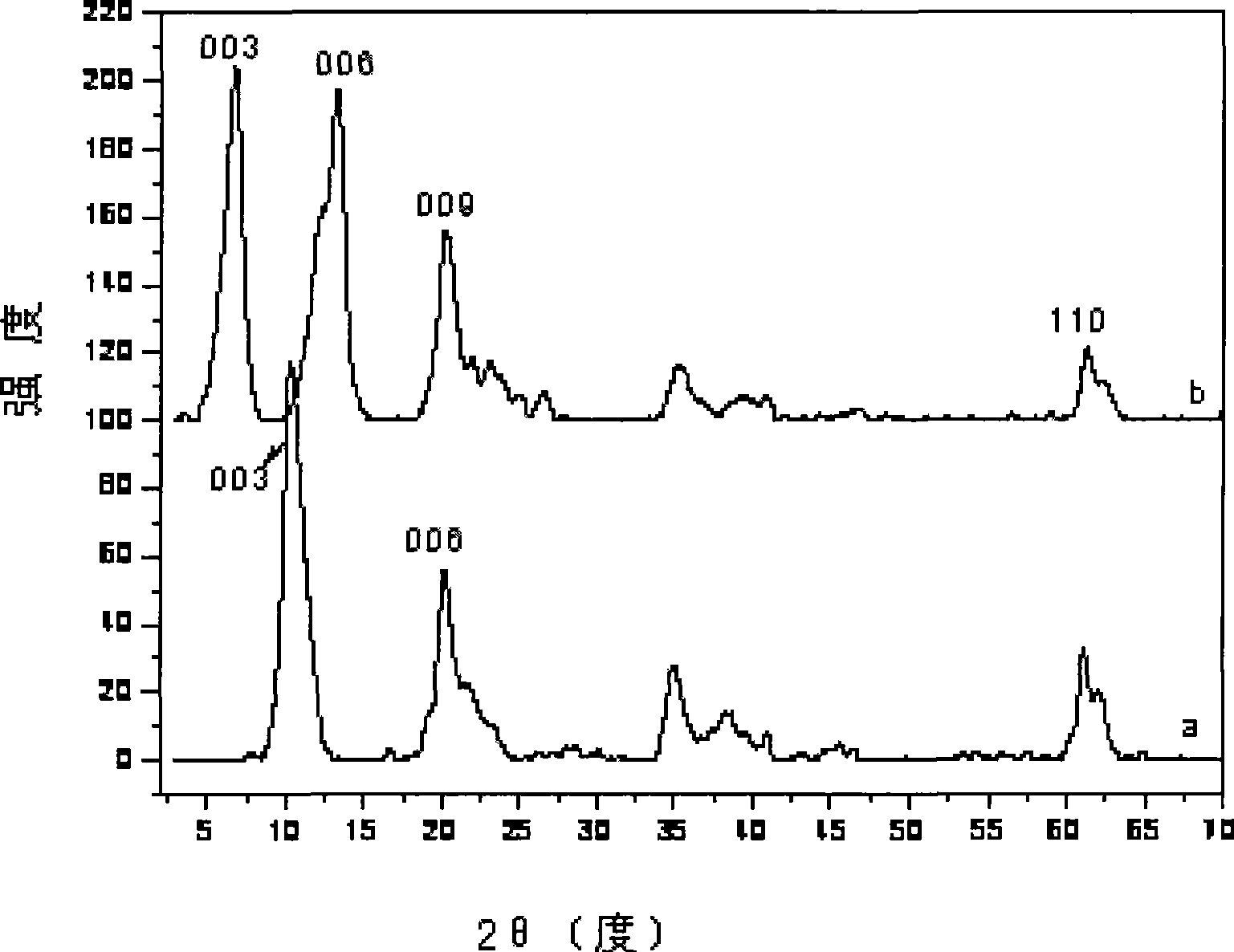
Image
Examples
Embodiment 1
[0027] A. Add 0.015mol of solid Mg(NO 3 ) 2 ·6H 2 O and 0.0075mol of solid Al(NO 3 ) 3 9H 2 O dissolved in 50mL to remove CO 2 Prepare a mixed salt solution of magnesium nitrate and aluminum nitrate in deionized water;
[0028] B. Dissolve 0.018mol carnosine and 0.0625mol NaOH in 50mL to remove CO 2 Prepare the mixed solution of carnosine and NaOH in deionized water;
[0029] C. Mix the nitrate solution prepared in step A under N 2 Slowly added dropwise to the mixed solution prepared in step B under protected conditions, stirred, and adjusted the pH range of the solution to 9 with 3mol / L NaOH;
[0030] D. Crystallizing the slurry obtained in step C at 60° C. for 24 hours;
[0031] E. The product is removed by CO 2 The deionized hot water was centrifuged and washed to neutrality, and dried at 70°C for 20 hours to obtain carnosine-intercalated hydrotalcite.
Embodiment 2
[0033] The slurry obtained in step C in Example 1 was transferred to a microwave rapid digestion tank, heated by microwave at 100° C. for 0.5 h, and the obtained product was used to remove CO 2 The deionized hot water was centrifuged and washed to neutral, and dried at 70°C for 24 hours to obtain carnosine-intercalated hydrotalcite.
Embodiment 3
[0035] Step A: take by weighing 15.384g Mg(NO 3 ) 2 ·6H 2 O and 11.2539g Al(NO 3 ) 3 9H 2 O dissolved in 100ml to remove CO 2 1. Prepare mixed salt solution with deionized water;
[0036] Step B: Dissolve another 7.2g NaOH in 100ml to remove CO 2 , deionized water;
[0037] Step C: Mix the nitrate solution prepared in step A under N 2 Slowly add dropwise to the lye prepared in step B under protected conditions, stir, adjust the pH range of the solution to 10 with 3mol / L NaOH, crystallize at 70°C for 40 hours, and remove CO 2 The deionized hot water is centrifugally washed to neutrality to obtain the nitrate hydrotalcite precursor;
[0038] Step D: dissolving carnosine in water, adding undried nitrate hydrotalcite precursor, the molar ratio of carnosine to aluminum ion is 1-3, and adjusting the pH to 8;
[0039] Step E: In 60 °C water bath and N 2 Ion exchange under protected conditions for 24 hours, the obtained product was used to remove CO 2 1. Centrifugal washing ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com