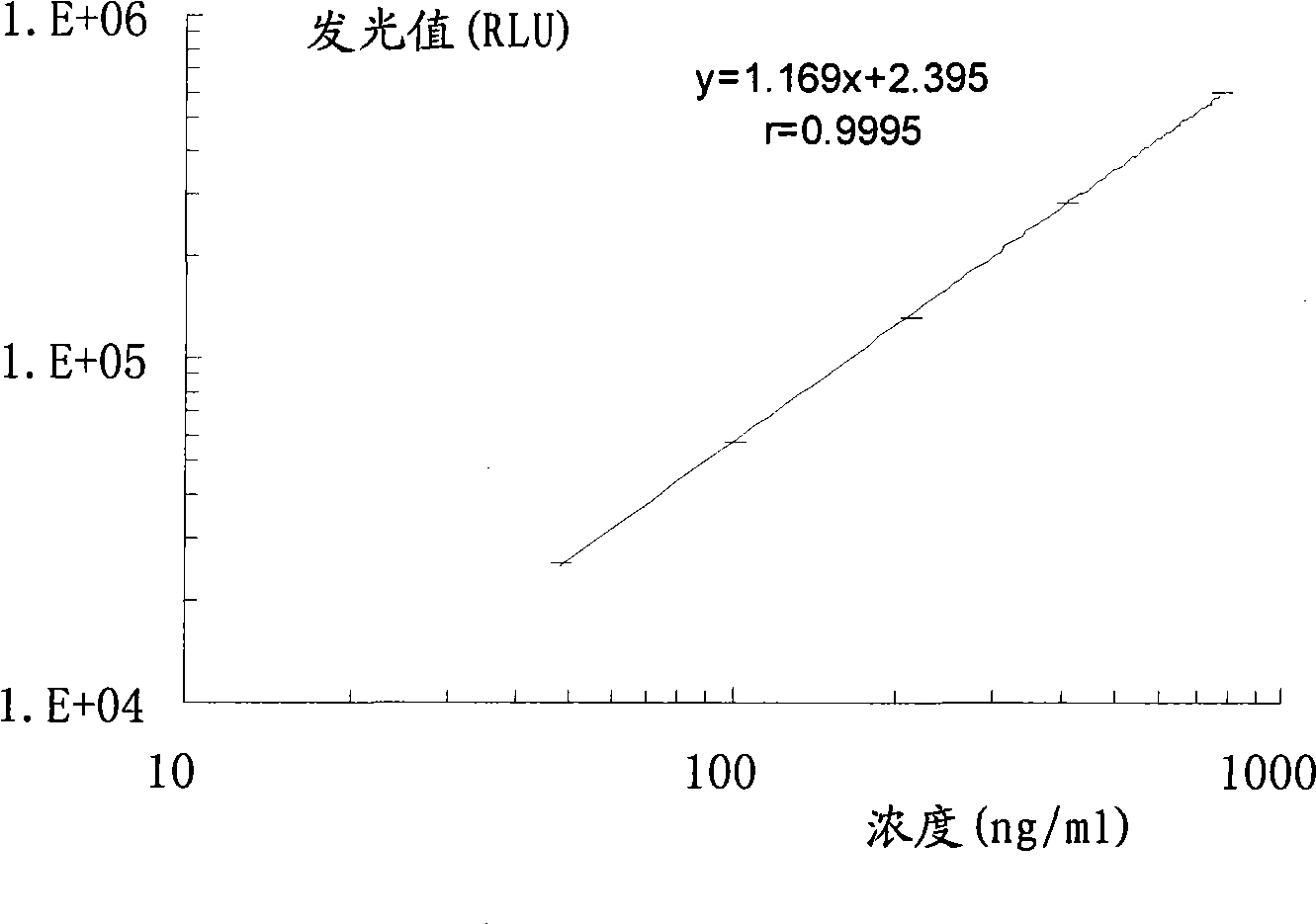
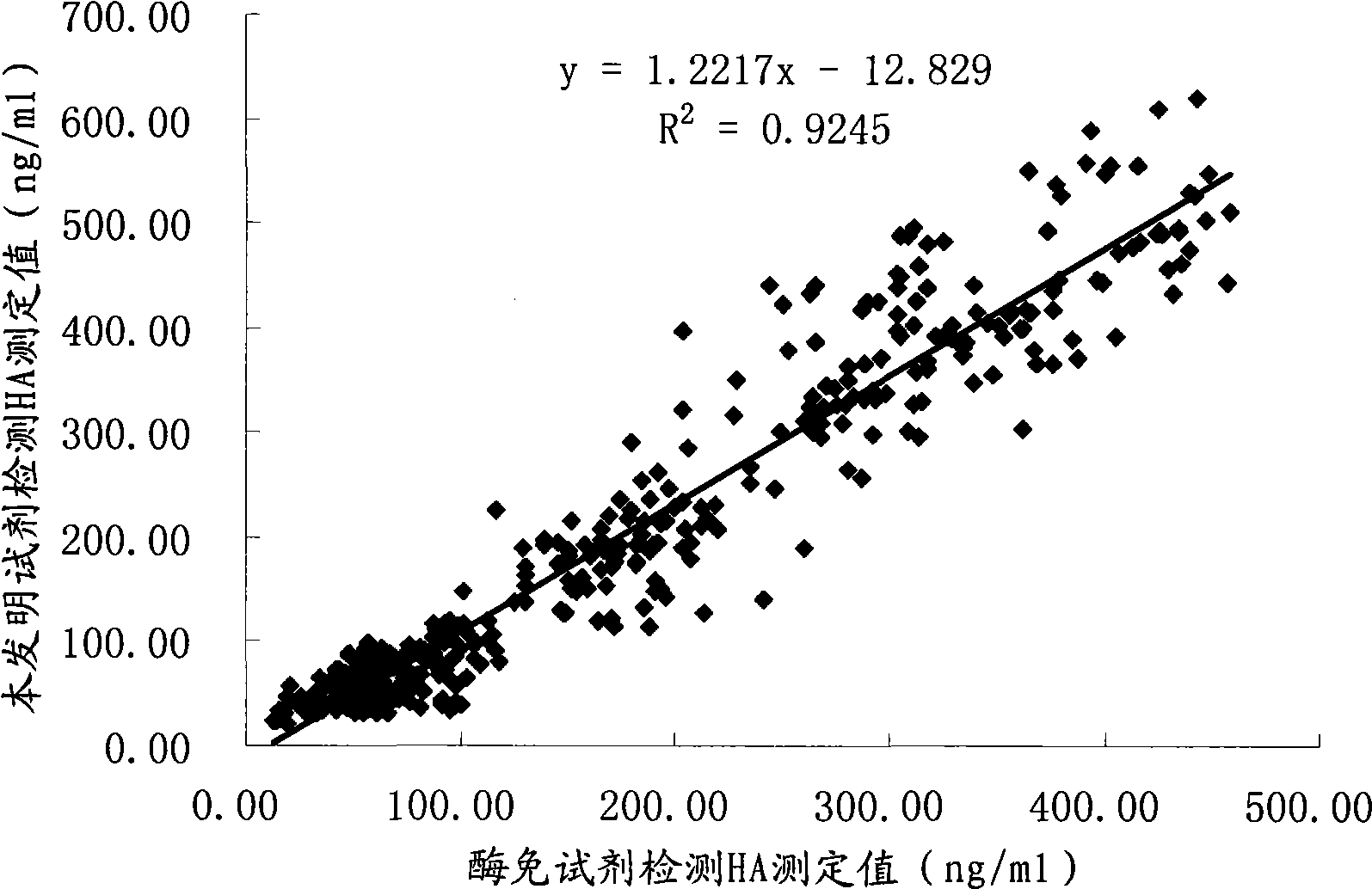
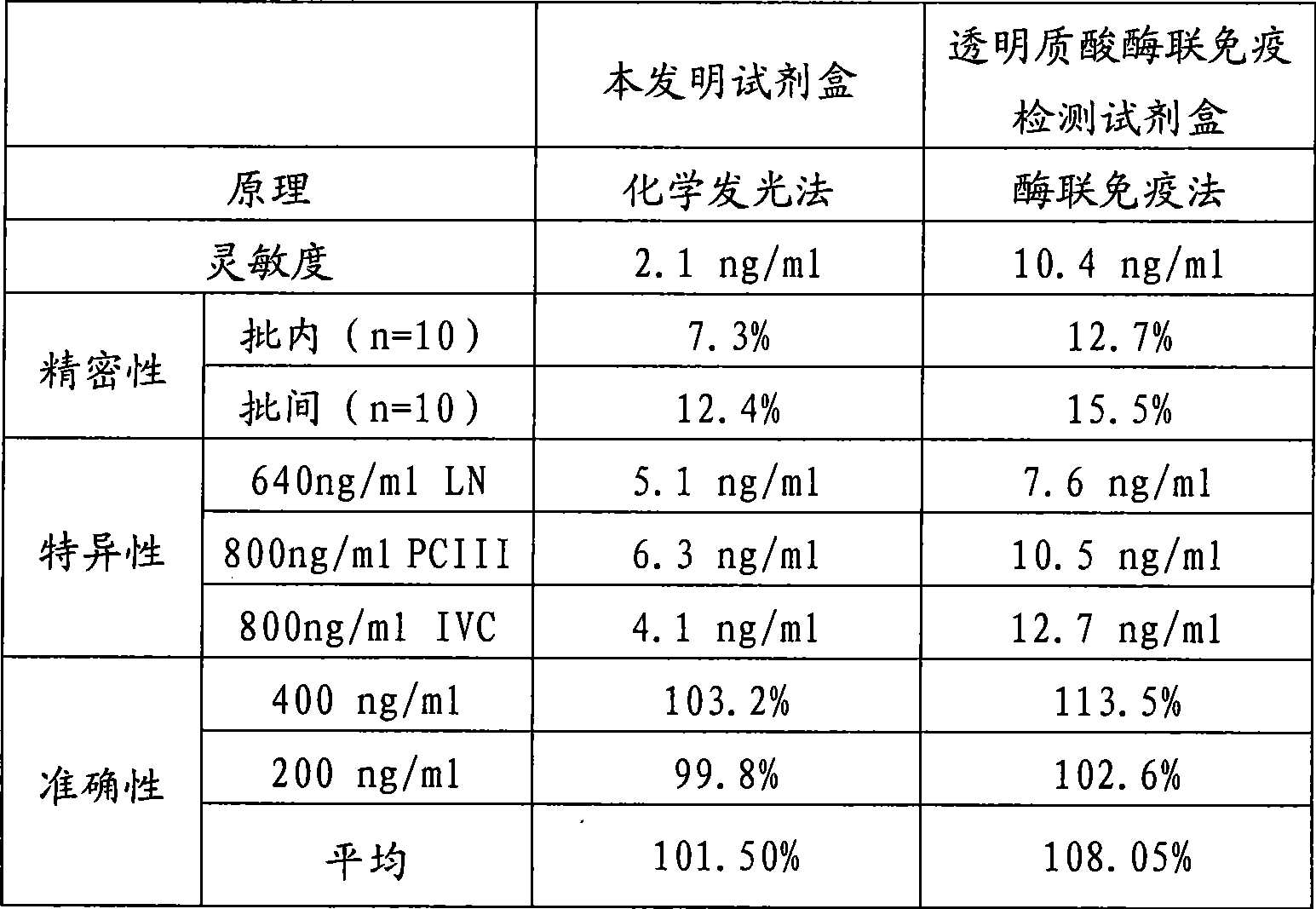
Chemoluminescent immunoassay kit of hyaluronic acid and preparation method thereof
A technology of uronic acid chemistry and hyaluronic acid, which is applied in the field of immunoassay medicine, can solve the problems of low sensitivity, poor precision, pollution hazards of radioactive waste and other problems of enzyme immunoassay reagents, and achieves the effect of ensuring sensitivity and improving sensitivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Preparation of Hyaluronic Acid Chemiluminescence Immunoassay Assay Kit of the present invention
[0030] 1. Preparation of enzyme-labeled hyaluronic acid-binding protein and biotin-conjugated antibody
[0031] 1. The hyaluronic acid-binding protein was coupled to horseradish peroxidase by the glutaraldehyde method or the improved sodium periodate method.
[0032] 2. Preparation of biotin-conjugated hyaluronic acid polyclonal antibody or monoclonal antibody
[0033] (1) Dissolve biotin (BNHS) in N, N-dimethylformamide (DMF) by conventional methods to make 1 mg / mL;
[0034] (2) Use 0.1mol / L NaHCO with a pH value of 9.0 3 Dilute the purified hyaluronic acid polyclonal antibody or monoclonal antibody to 1-2 mg / mL;
[0035] (3) Mix according to the volume ratio of BNHS and antibody is about 1:8 or the weight ratio is about 1:7, and react for 2-4 hours under stirring at room temperature;
[0036] (4) Put it into 0.05 mol / L PBS with a pH value of 7.2, dialyze ove...
Embodiment 2-3
[0090] Example 2-3 Preparation of Hyaluronic Acid Chemiluminescence Immunoassay Assay Kit of the present invention
[0091] The hyaluronic acid chemiluminescent immunoassay assay kit was prepared in the same manner as in Example 1, except that plastic beads and plastic tubes were used as carriers.
Embodiment 4
[0092] Example 4 Preparation of Hyaluronic Acid Chemiluminescence Immunoassay Assay Kit of the present invention
[0093] Except that the magnetic particles were used as the carrier and the magnetic particle immobilized avidin was prepared by the EDC coupling method, the hyaluronic acid chemiluminescence immunoassay assay kit was prepared in the same manner as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com