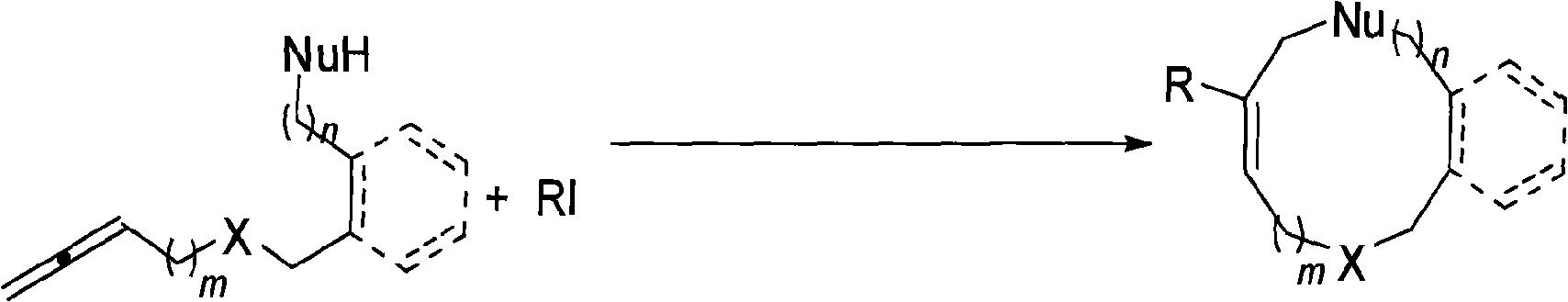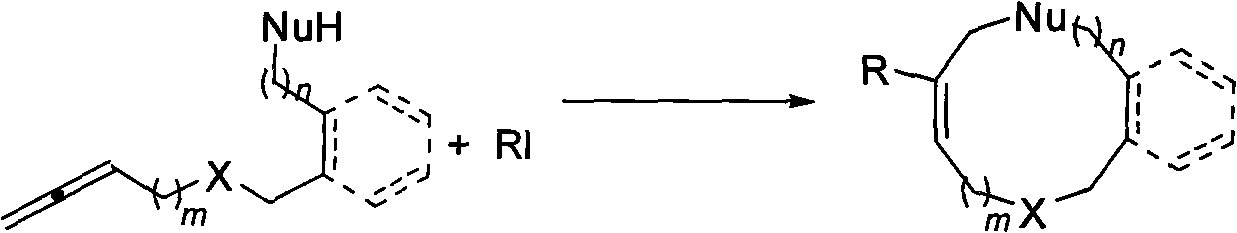A method for synthesis of medium-ring compounds of 9 to 12 rings
A technology for cyclic compounds and iodides, applied in the field of synthesizing 9-12-membered mid-ring compounds, can solve the problems of reduced yield, high production cost, strict operation requirements, etc., and achieves easy separation and purification, strong substrate universality, and reaction simple conditional effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020]
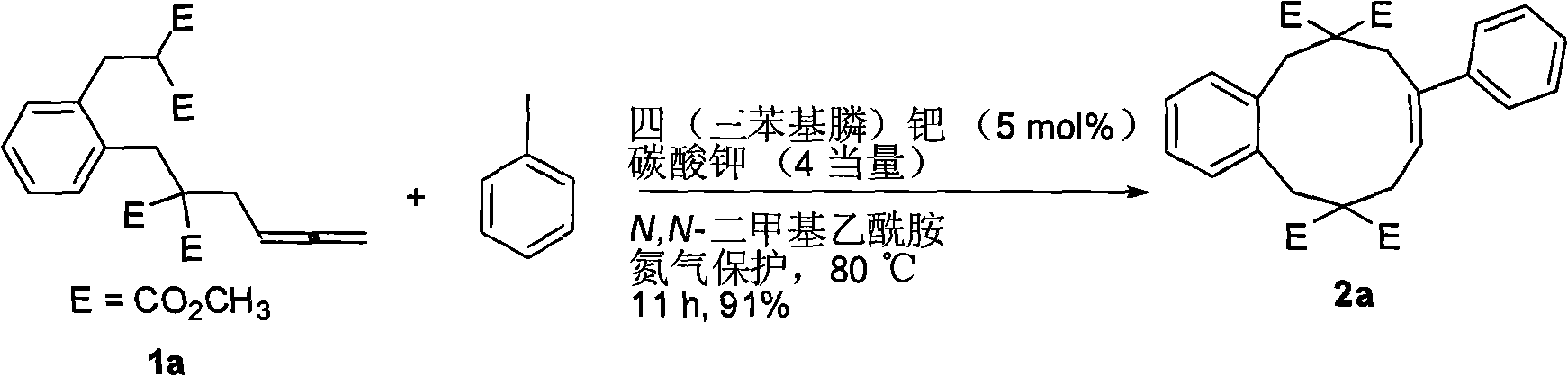
[0021] Potassium carbonate (55.6mg, 0.4mmol) was added to the reaction tube, the nitrogen was replaced three times, and tetrakis(triphenylphosphine)palladium (5.7mg, 0.005mmol), 3-(2-(2, 2-Bis(methoxycarbonyl)-4,5-dihexenyl)phenyl)-2-(methoxycarbonyl)propanoic acid methyl ester 1a (41.8mg, 0.1mmol), iodobenzene (40.9mg, 0.2mmol ), and N,N-dimethylacetamide (1.5 mL). After reacting at 80°C for 11 hours, the reaction was first quenched with 10 mL of water, and then extracted with ether (30 mL×3). The ether layers were combined, washed with water and brine, and dried over anhydrous sodium sulfate. Filtration, spin-drying, and silica gel column separation (petroleum ether / ethyl acetate / dichloromethane=5 / 1 / 1) to obtain the product (E)-8-phenyl-6,6,11,11-(tetramethoxy Carbonyl)benzo-8-cyclodecene (44.9 mg, 91%) 2a. The product is a white solid, melting point; 204-205°C (dichloromethane / n-hexane).
[0022] 1 H NMR (300MHz, CDCl 3 )δ7.34-7.18(m, 5H), 7.18-7.08(m, 2H),...
Embodiment 2
[0024]
[0025] According to the method described in Example 1, potassium carbonate (110.3mg, 0.8mmol), tetrakis (triphenylphosphine) palladium (11.6mg, 0.01mmol), 1a (83.0mg, 0.2mmol), p-methoxycarbonyl iodobenzene (103.9mg, 0.4mmol) was reacted in N,N-dimethylacetamide (3mL) to give product 2b (92.0mg, 84%) as a white solid with a melting point of 201-202°C (dichloromethane / n-hexane) .
[0026] 1 H NMR (300MHz, CDCl 3 )δ7.95(d, J=8.3Hz, 2H), 7.30(d, J=8.3Hz, 2H), 7.18-7.09(m, 2H), 6.91-6.78(m, 2H), 5.23(dd, J =13.5Hz, J=4.7Hz, 1H), 3.90(s, 3H), 3.87(s, 3H), 3.84(s, 3H), 3.80(s, 3H), 3.58(d, J=14.7Hz, 1H ), 3.45(d, J=15.0Hz, 1H), 3.26(d, J=14.4Hz, 1H), 3.17(d, J=14.7Hz, 2H), 2.96(d, J=15.0Hz, 1H), 2.87(s, 3H), 2.63(dd, J=13.5Hz, J=3.9Hz, 1H), 2.45(t, J=13.5Hz, 1H); 13 C NMR (75MHz, CDCl 3 ): δ172.0, 171.7, 171.1, 170.7, 166.7, 147.1, 139.5, 135.4, 135.2, 130.9, 129.8, 129.4, 129.1, 128.8, 127.6, 127.1, 127.0, 58.5, 57.8, 52.8, 52.5, 2 51.8, 33.1, 32.4, 31.0, 30.3; I...
Embodiment 3
[0028]
[0029] According to the method described in Example 1, potassium carbonate (109.6mg, 0.8mmol), tetrakis (triphenylphosphine) palladium (11.4mg, 0.01mmol), 1a (82.8mg, 0.2mmol), 4-acetyl iodobenzene (98.4mg, 0.4mmol) was reacted in N,N-dimethylacetamide (3mL) to give product 2c (94.3mg, 89%) as a white solid, melting point: 156-158°C (dichloromethane / n-hexane) .
[0030] 1 H NMR (300MHz, CDCl 3)δ7.87(d, J=7.8Hz, 2H), 7.31(d, J=7.8Hz, 2H), 7.17-7.05(m, 2H), 6.90-6.76(m, 2H), 5.24(dd, J =13.0Hz, J=4.2Hz, 1H), 3.86(s, 3H), 3.83(s, 3H), 3.79(s, 3H), 3.57(d, J=14.7Hz, 1H), 3.44(d, J =15.0Hz, 1H), 3.25(d, J=14.7Hz, 1H), 3.16(d, J=14.7Hz, 2H), 2.96(d, J=14.7Hz, 1H), 2.86(s, 3H), 2.68-2.55(m, 4H), 2.44(t, J=13.0Hz, 1H); 13 C NMR (75MHz, CDCl 3 ): δ197.5, 171.9, 171.6, 171.1, 170.7, 147.3, 139.4, 135.7, 135.3, 135.2, 131.0, 129.8, 129.4, 127.84, 127.76, 127.1, 127.0, 58.5, 57.8, 55.8, 65. 33.1, 32.4, 30.9, 30.2, 26.6; IR (KBr, cm -1 ): 3036, 3003, 2955, 2845, 1733, 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com