Preparation method of S-(+)-o-chlorobenzoyl glycine
A technology of o-chlorophenylglycine and o-chlorobenzaldehyde is applied in the preparation of organic compounds, chemical instruments and methods, cyanide reaction preparation, etc., and can solve the problems of low actual yield, high raw material cost, long process route and the like, Achieve the effect of low cost of raw materials, less use of organic solvents and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
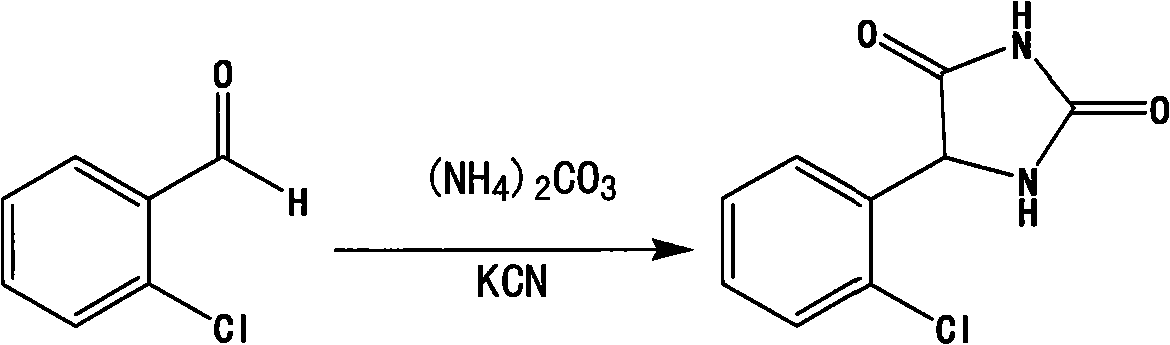
[0019] A preparation method of (±)-o-chlorophenylhydantoin, the synthetic route is as follows:
[0020]
[0021] The specific process of the preparation method is: 5.6g of o-chlorobenzaldehyde, 2.6g of potassium cyanide and 9.1g of ammonium carbonate are added to 50ml of 50% ethanol aqueous solution under stirring, and the temperature is raised to 55-65°C. , kept stirring and reacting for 24 hours, cooled to room temperature, adjusted pH 2 to 3 with hydrochloric acid, filtered, washed with water, and dried to obtain (±)-o-chlorophenylhydantoin crude product, which was recrystallized with water to obtain 6.8 g of white crystals. The yield is 80%, m.p175~177°C, and the purity (HPLC) is 99.80%.
Embodiment 2
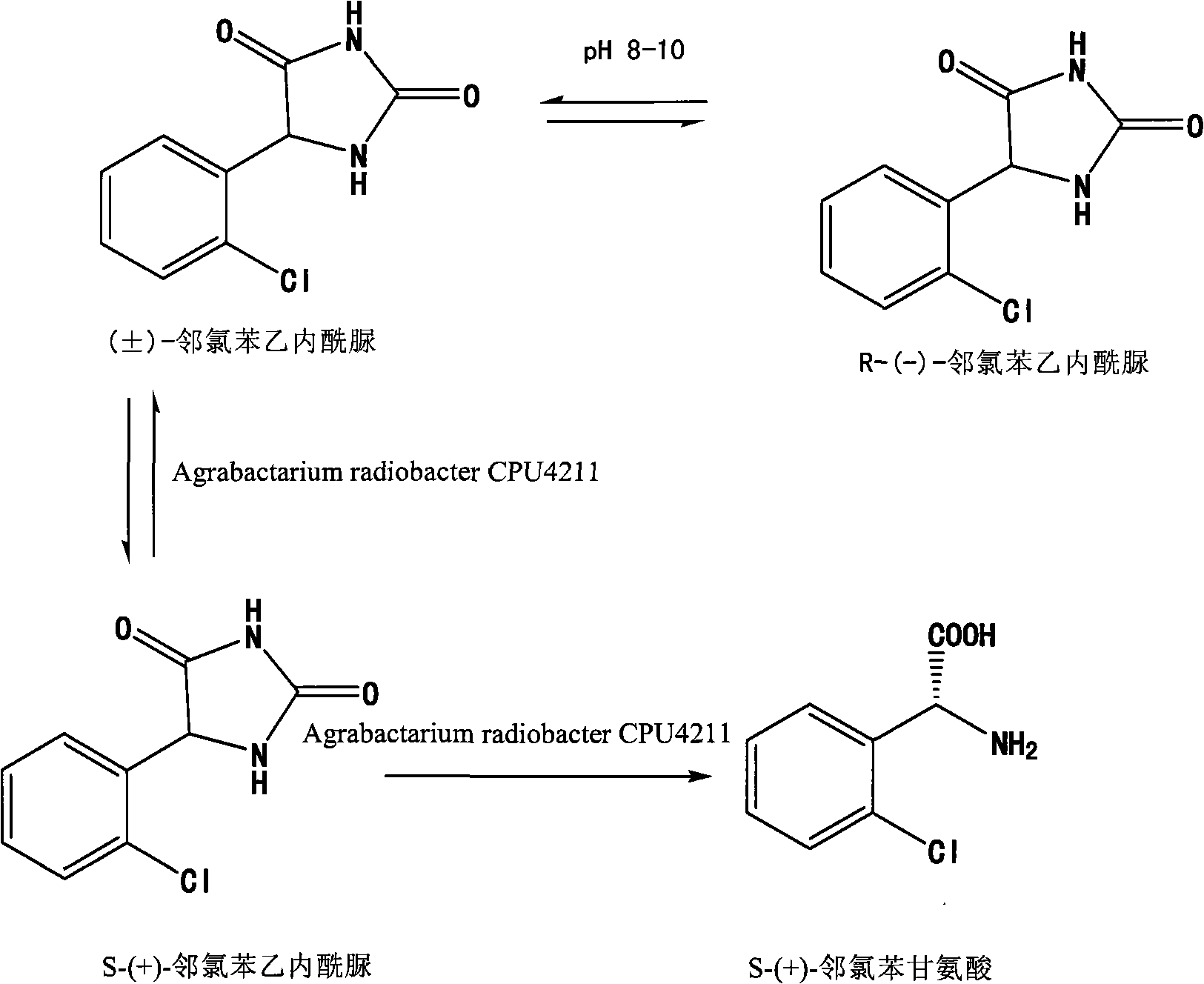
[0023] A kind of preparation method of S-(+)-o-chlorophenylglycine formic acid, the reaction involved in it is shown in the following formula:
[0024]
[0025] The specific process of the preparation method is as follows: 6.8g of the crystallization of (±)-o-chlorophenylhydantoin obtained in Example 1 and 100ml of water are stirred and mixed evenly, and an aqueous sodium hydroxide solution is added to adjust the pH to 8.5, and Agrobacterium Agrabacterium radiobacter CPU4211 bacterium (provided by sigma-aldrich company) 1g, under nitrogen protection and 40 ℃, shake reaction for 42 hours, centrifuge, discard the precipitate, after the filtrate is decolorized with activated carbon, add 200ml dichloromethane, stir and At 0-5°C, adjust the pH to 4-5 with hydrochloric acid, separate the dichloromethane layer, and extract the water layer twice with 100ml of dichloromethane. The dichloromethane layers were combined, dried over anhydrous magnesium sulfate, and concentrated to dryne...
Embodiment 3
[0027] A kind of preparation method of S-(+)-o-chlorophenylglycine methyl ester, its specific process is as follows: the crystallization of (±)-o-chlorophenylhydantoin and 1000ml water of 70g embodiment 1 gained are stirred and mixed, Add aqueous sodium hydroxide solution to adjust the pH to 9, add 12 g of Agrabactarium radiobacter CPU4211 cells (provided by sigma-aldrich company), shake the reaction for 48 hours under nitrogen protection and a temperature of 40 ° C, centrifuge, discard the precipitate, After the filtrate was decolorized with activated carbon, 200ml of dichloromethane was added, and the pH was adjusted to 4-5 with hydrochloric acid under stirring at a temperature of 0-5°C. The dichloromethane layer was separated, and the aqueous layer was extracted twice with 1000ml of dichloromethane. The dichloromethane layers were combined, dried over anhydrous magnesium sulfate, and concentrated to dryness under reduced pressure to obtain 54 g of white solid, namely S-(+)-o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com