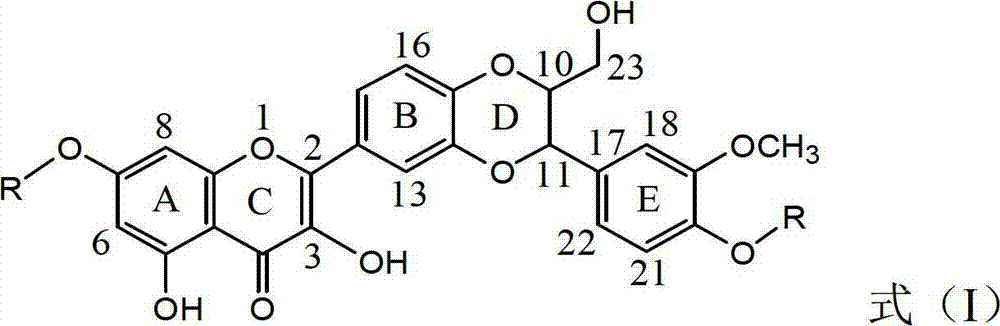
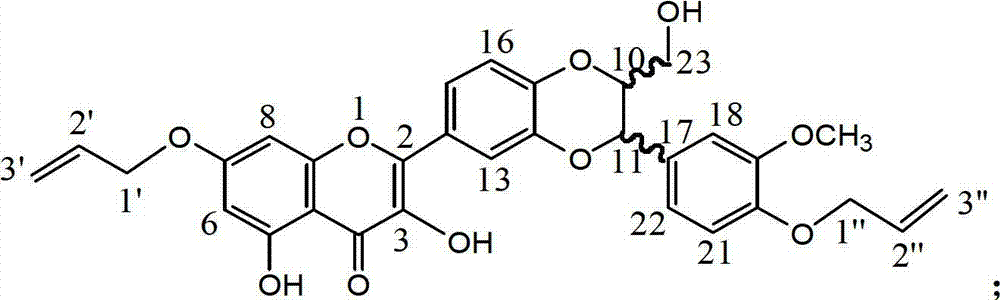
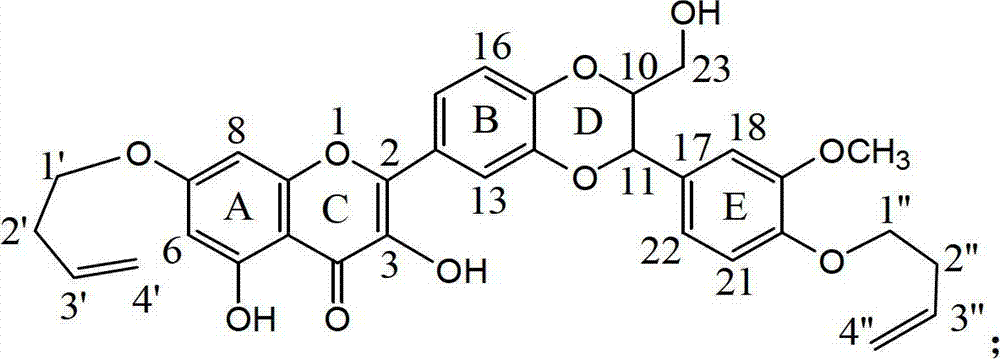
7 and 20 dehydro-silybin dialky ether and preparation method and medicine use thereof
A technology of silibinin and dehydrogenated water, which is applied in the direction of drug combinations, pharmaceutical formulas, and medical preparations containing active ingredients, can solve the problems of changes and damage of the biphospholipid layer of the cell membrane, and achieve a strong anti-lipid overload. Oxidation, simplicity of the method, and the effect of protecting the metal ion chelating ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 : the preparation of compound I-a i.e. 7,20-bisallyl-dehydrosilibinin ether
[0031]
[0032] In a dry reaction flask, 0.240 g of silibinin was dissolved in 4 ml of DMF, 0.276 g of potassium carbonate was added, and stirred for 10 minutes to dissolve completely. 0.125 g of allyl bromide was slowly added dropwise, stirred for 10 minutes, and then heated at 75°C for 3 hours. Stand to cool, add 20 ml of distilled water, extract with ethyl acetate three times (10 ml each time), combine the organic layers, wash with 20 ml of distilled water, dry over anhydrous sodium sulfate, and concentrate under reduced pressure. The brown-yellow crude product was obtained, and it was eluted with 200-300 mesh silica gel (10 g) with chloroform: ethyl acetate: acetic acid = 20:1:0.1 to obtain 74.3 mg of yellow crystal (I-a). Yield 26.6%.
[0033] Compound I-a name: 2-[2,3-dihydro-3-(4-allyloxy-3-methoxyphenyl)-2-hydroxymethyl-1,4-benzodioxane -6-]-7-(allyloxy)-3,5,-dihydroxy...
Embodiment 2
[0034] Example 2 : Compound I-b is the preparation of 7,20-bis(1-butenyl)-dehydrosilibinin ether
[0035] In a dry reaction flask, 0.240 g of silibinin was dissolved in 4 ml of DMF, 0.276 g of potassium carbonate was added, and stirred for 10 minutes to dissolve completely. 0.135 g of 4-bromo-1-butene was slowly added dropwise, stirred for 10 minutes, and then heated at 75°C for 3 hours. Stand to cool, add 20 ml of distilled water, extract with ethyl acetate three times (10 ml each time), combine the organic layers, wash with 20 ml of distilled water, dry over anhydrous sodium sulfate, and concentrate under reduced pressure. The brownish-yellow crude product was subjected to column chromatography on 200-300 mesh silica gel (10 g), eluted with chloroform: ethyl acetate: acetic acid = 20:1:0.1, and 70 mg of yellow crystal (I-b) was obtained. Yield 23.8%.
[0036] Compound I-b name: 2-[2,3-dihydro-3-(4-enbutyloxy-3-methoxyphenyl)-2-hydroxymethyl-1,4-benzodioxane -6-]-7-(en...
Embodiment 3
[0037] Example 3 : Compound I-c is 7, the preparation of 20-bis(2-methyl-2-ene butyl)-dehydrosilibinin ether
[0038]
[0039] In a dry reaction flask, 0.48 g of silibinin was dissolved in 6 ml of DMF, 0.55 g of potassium carbonate was added, and stirred for 10 minutes to dissolve completely. 0.33 g of isopentenyl bromide was slowly added dropwise, stirred for 10 minutes, and then heated to reflux for 2 hours. Stand to cool, add 20 ml of distilled water, extract with ethyl acetate three times (10 ml each time), combine the organic layers, wash with 10 ml of distilled water, dry over anhydrous sodium sulfate, and concentrate under reduced pressure. The brownish-yellow crude product was obtained and subjected to column chromatography on 200-300 mesh silica gel (20 g), eluting with chloroform: ethyl acetate: acetic acid = 7:1:0.1 to obtain 220 mg of yellow crystal (I-c). Yield 35.6%.
[0040]Compound I-c name: 2-{2,3-dihydro-3-[4-(2-methyl-2-enebutyloxy)-3-methoxyphenyl]-2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com