Pure organic dye adopting multiple heterocycles and derivants thereof as conjugated unit and dye-sensitized solar cell prepared thereby
A technology of organic dyes and derivatives, applied in the field of dye-sensitized solar cells, can solve the problems of high price, limited practical application of precious metal resources, weak absorption spectrum, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] Example 1: Synthesis of pure organic dye of chemical structure C
[0097] The synthetic route is as follows:
[0098]
[0099] Synthesis of material with structural formula 11:
[0100] In the reactor, 5.00 g of 3,4-dimethoxythiophene (34.67 mmol), 32.65 g of ethanedithiol (34.46 mmol) and 0.50 g of p-toluenesulfonic acid (2.62 mmol) were dissolved in 100 In 1 ml of toluene, protected by argon, the temperature was raised to reflux for 72 hours. After cooling to room temperature, add 100 ml of water, extract the aqueous phase with 100 ml of ether three times, wash the organic phase with 100 ml of 5% sodium hydroxide aqueous solution, combine the organic phases, dry with anhydrous magnesium sulfate, spin off the solvent, and distill under reduced pressure to obtain Product 11. Yield: 45%.
[0101] Synthesis of materials with structural formula 12:
[0102] In the reactor, 2.66 g of product 11 was dissolved in a mixed solvent of 2.52 ml of N'N-dimethylformamide and 40 ml of ...
Embodiment 2
[0124] Example 2: Dye-sensitized solar cell prepared with pure organic dye of chemical structure C
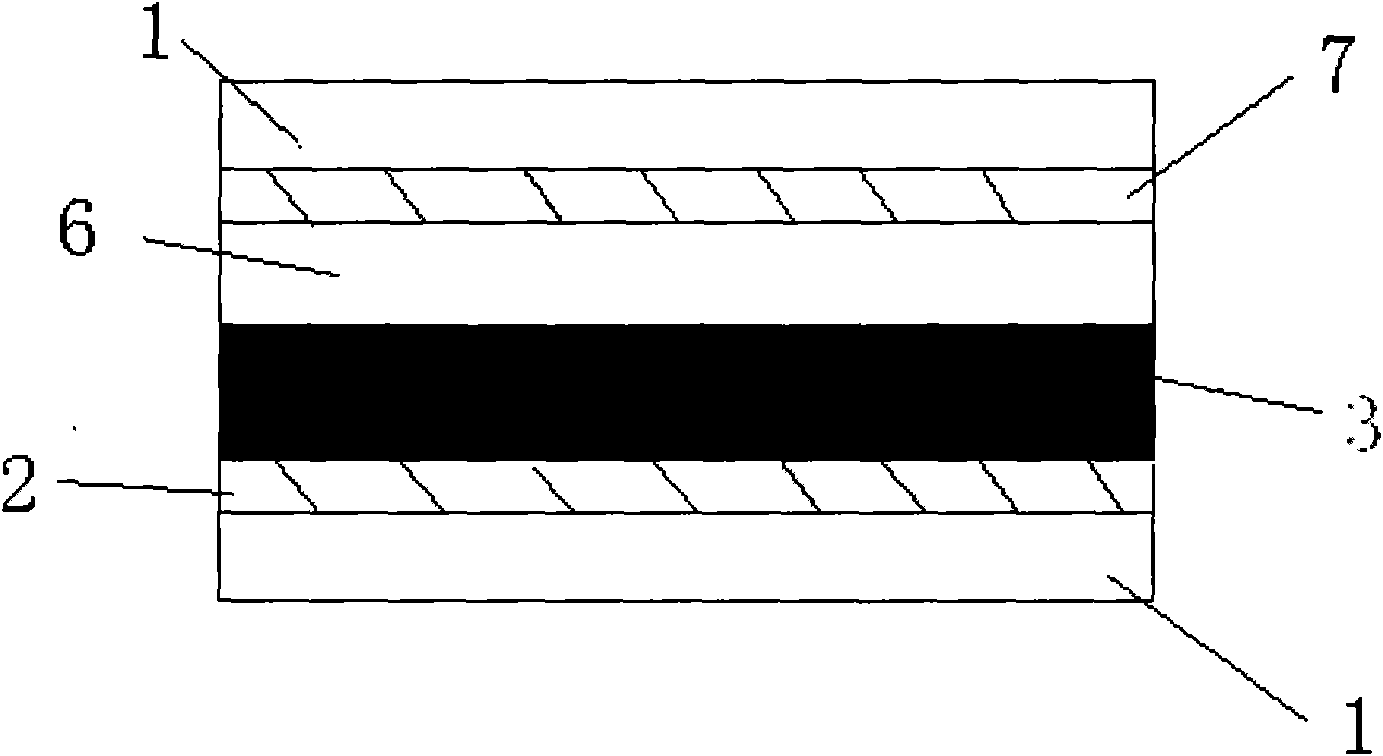
[0125] The photoanode (light absorption layer) of the dye-sensitized solar cell adopts a mesoporous double layer, and the bottom layer is made of 20nm TiO 2 It is composed of nanocrystals, the thickness is 7μm, and the upper film thickness is 400nm TiO 2 It is composed of light scattering particles and has a thickness of 5μm. Preparation of TiO 2 Nanocrystalline and TiO 2 For the method of nanostructured double-layer membrane electrode, please refer to the article (Wang P. et al., Enhance the Performance of Dye-Sensitized Solar Cells by Co-grafting AmphiphilicSensitizer and Hexadecylmalonic Acid on TiO 2 Nanocrystals, J. Phys. Chem. B., 107, 2003, 14336).
[0126] The prepared TiO 2 The nanostructured double-layer membrane electrode is immersed in acetonitrile / tert-butanol containing 300μM chemical structure C dye and 300μM Cheno (3,7-dihydroxy-4-cholic acid) for 12 hours. At this ...
Embodiment 3
[0128] Example 3: Synthesis of pure organic dye of chemical structure D and dye-sensitized solar cell prepared therefrom
[0129]
[0130] The pure organic dyes of the chemical structure D used are raw materials 3,4-ethylenedioxythiophene, furan and 3,4-ethylenedithiothiophene, synthesized by the steps and conditions of Example 1.
[0131] NMR data of pure organic dyes of chemical structure D:
[0132] 1 H NMR(400MHz, DMSO, δ H ): 13.75(s, 1H), 8.28(s, 1H), 7.52(d, 1H), 7.23(d, 1H), 7.01(m, 4H), 6.91(m, 4H), 6.85(d, 1H) , 6.80 (d, 2H), 4.44 (d, 2H), 4.38 (d, 2H), 3.82 (d, 4H), 3.46 (s, 4H), 1.67 (m, 2H), 1.30-1.41 (m, 16H) ), 0.88-0.90 (m, 12H).
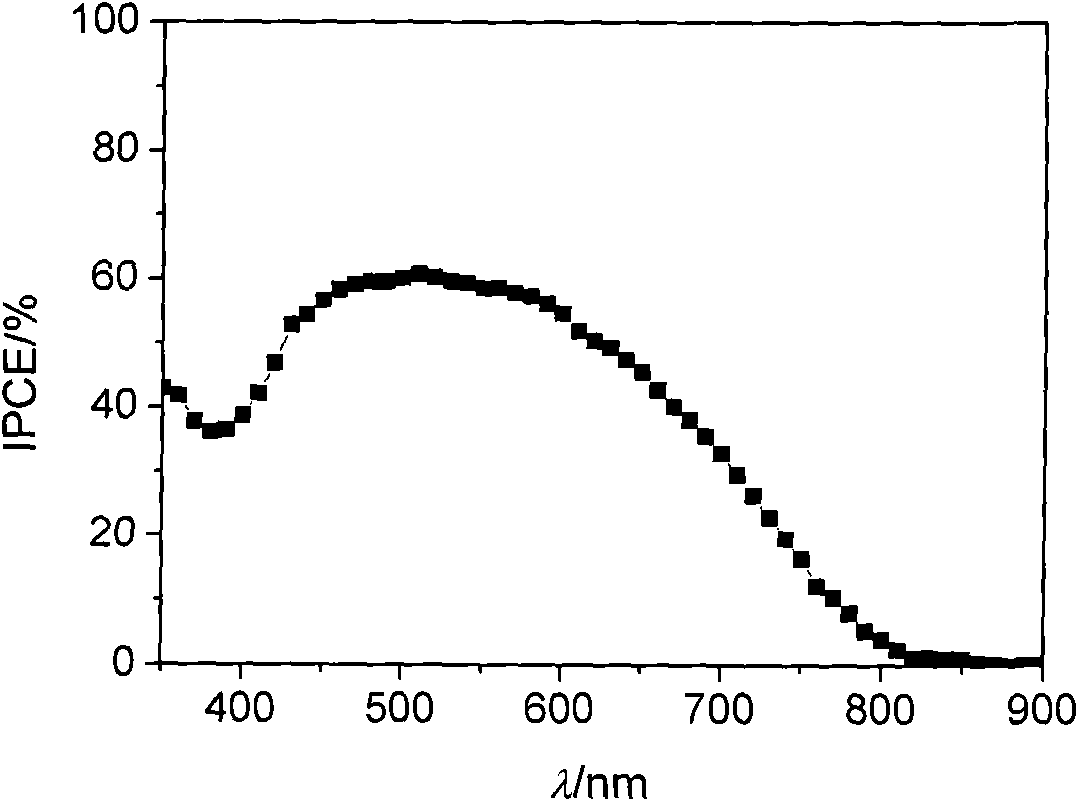
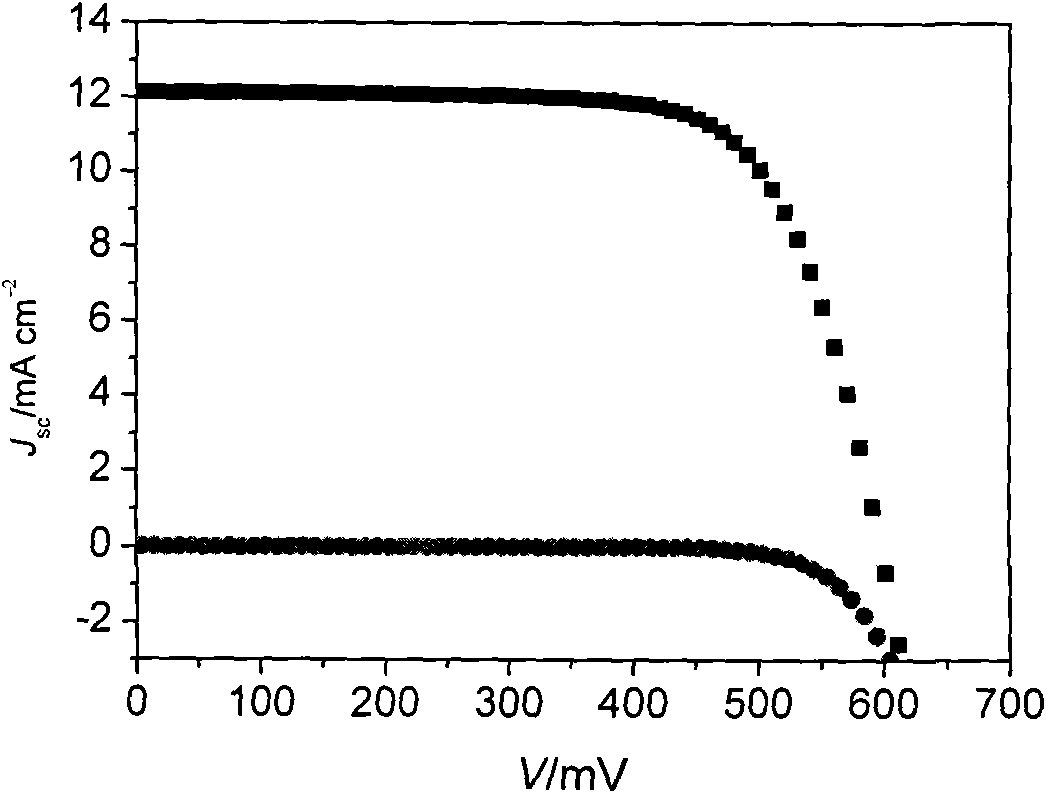
[0133] The dye-sensitized solar cell was prepared according to the method of Example 2, and the parameters of the obtained dye-sensitized solar cell are shown in the attached table of the specification.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Short circuit photocurrent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com