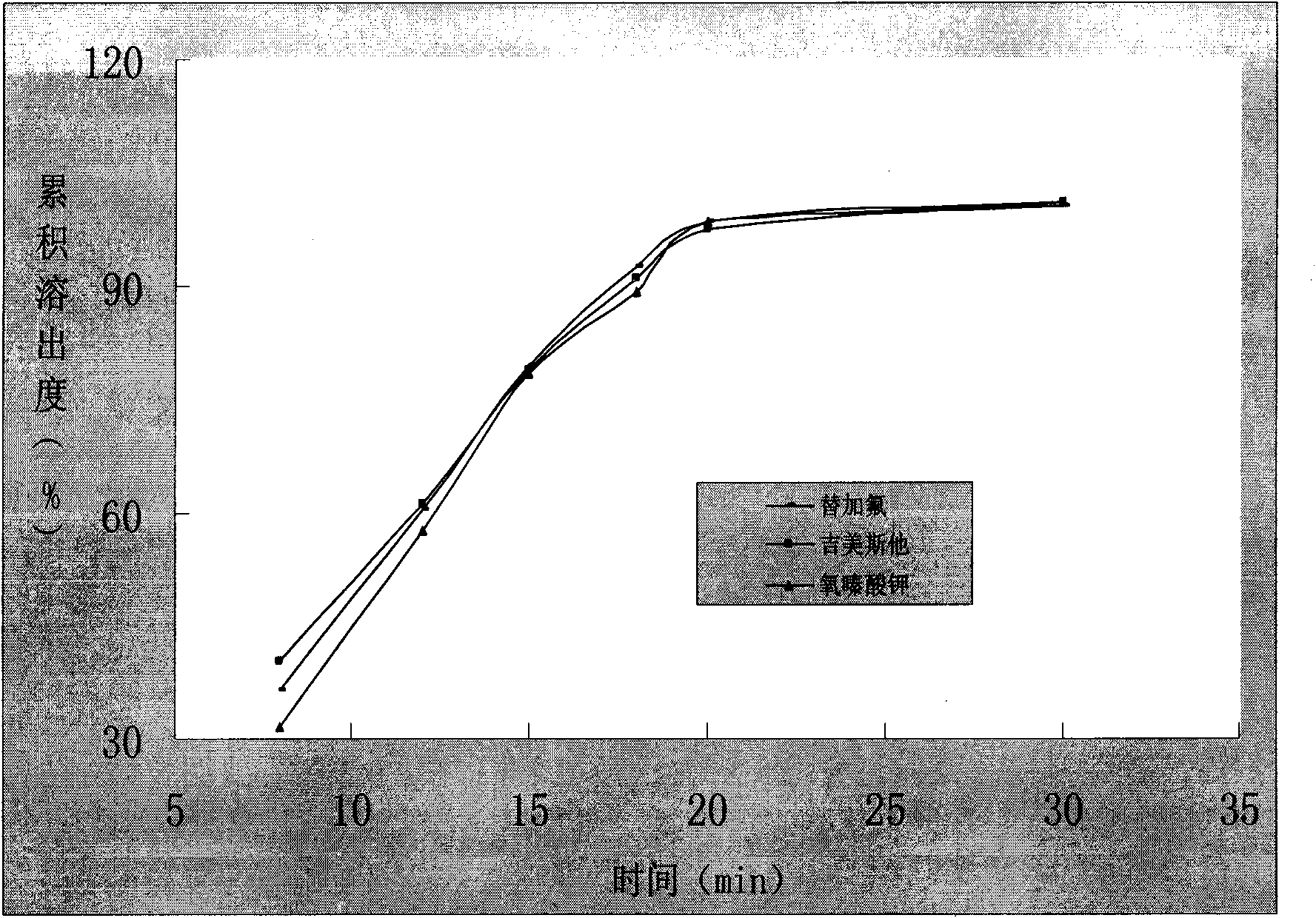
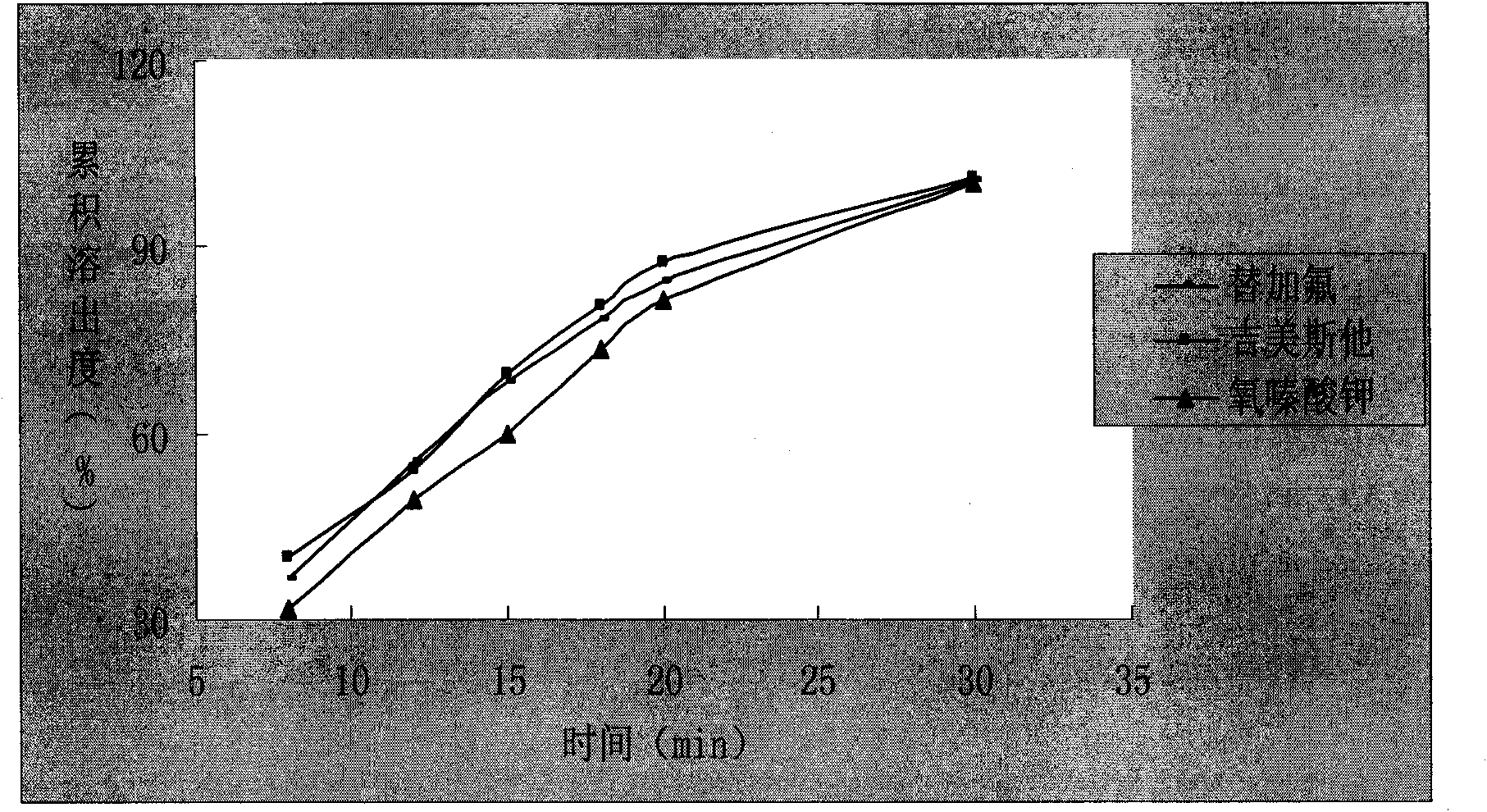
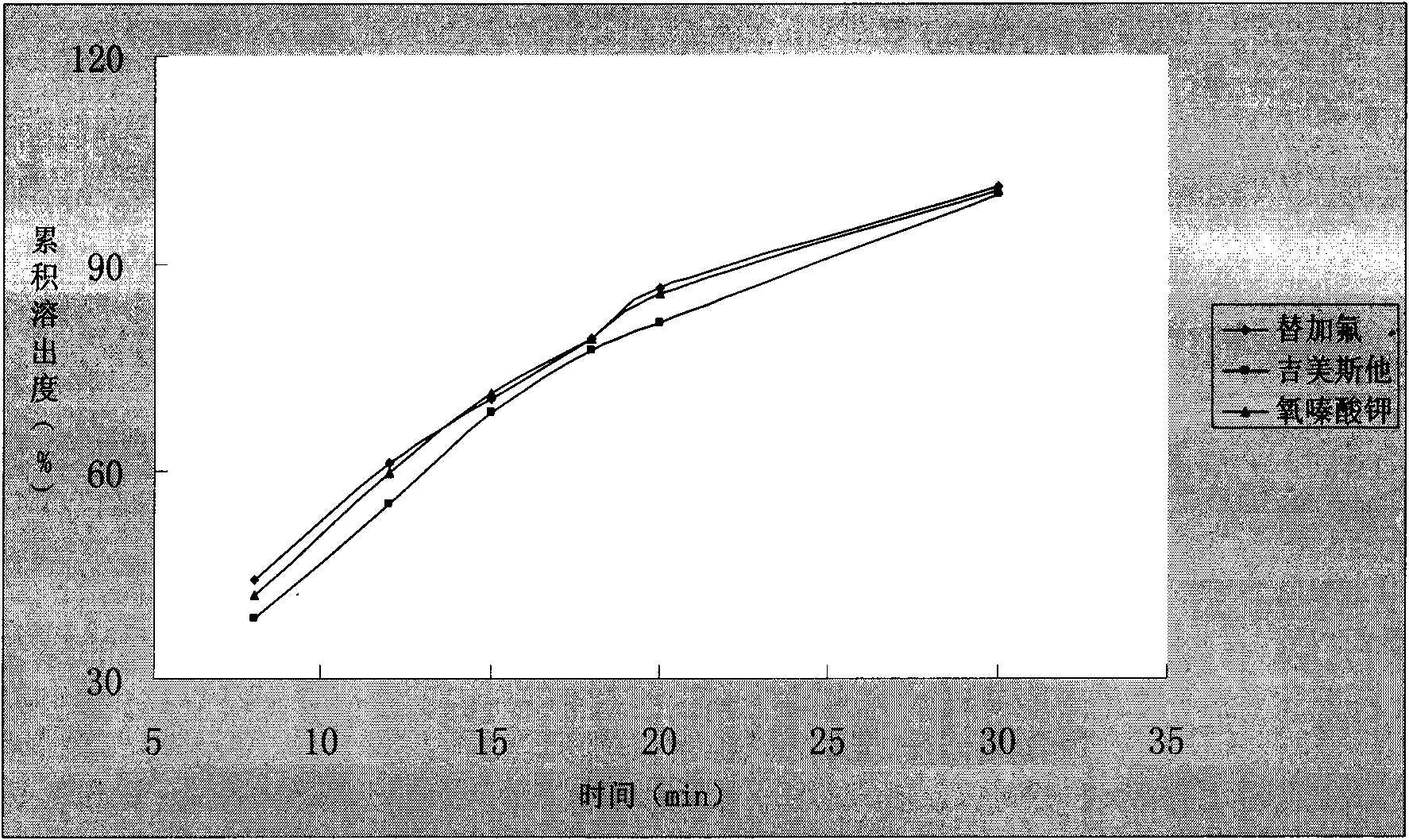
Capsule preparation containing tegafur, Jimeisita and Potassium Oxonate and preparation method thereof
A technology of potassium oxonate and tegafur, applied in the field of medicine, can solve problems such as instability and poor water solubility, and achieve the effects of stable dissolution rate, fast dissolution rate and improved bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1 The preparation of this Fategafur, gemesta and oxonate potassium capsule preparations:
[0045] Name of raw material Amount / g
[0046]
[0047] Prescription for tegafur pellets:
[0048] Tegafur 20
[0049] Microcrystalline Cellulose 22
[0050] Appropriate amount of purified water
[0051] Prescription of gemesstat pellets:
[0052] Gimesta 5.25
[0053] starch 10
[0054] Appropriate amount of purified water
[0055] Prescription of gemesstat pellets:
[0056] Potassium oxonate 15..5
[0057] Starch 15
[0058] Appropriate amount of purified water
[0059] other:
[0060] Sodium p-dodecylbenzenesulfonate 10
[0061]
[0062] A total of 1000 capsules were made
[0063] Preparation Process:
[0064] (1) Pass tegafluoride through a 100-mesh sieve, add auxiliary materials and mix evenly, add an appropriate amount of purified water to prepare soft ma...
Embodiment 2
[0067] Embodiment 2 The preparation of this Fategafur, gemesta and oxonate potassium capsule preparations:
[0068] Name of raw material Amount / g
[0069]
[0070] Prescription for tegafur pellets:
[0071] Tegafur 25
[0072] Microcrystalline Cellulose 20
[0073] 2% HPMC aqueous solution appropriate amount
[0074] Prescription of gemesstat pellets:
[0075] Guimesta 7.25
[0076] Microcrystalline Cellulose 12
[0077] 2% HPMC aqueous solution appropriate amount
[0078] Prescription of gemesstat pellets:
[0079] Potassium oxonate 24.5
[0080] Microcrystalline Cellulose 14
[0081] 2% HPMC aqueous solution appropriate amount
[0082] other:
[0083] Sodium Lauryl Sulfate 15
[0084]
[0085] A total of 1000 capsules were made
[0086] Preparation Process:
[0087] (1) Pass tegafur through a 100-mesh sieve, add auxiliary materials and mix evenly, add an approp...
Embodiment 3
[0090] Embodiment 3 The preparation of this Fategafur, gemesta and oxonate potassium capsule preparations:
[0091] Name of raw material Amount / g
[0092]
[0093] Prescription for tegafur pellets:
[0094] Tegafur 25
[0095] Pregelatinized starch 15
[0096] Lactose 12
[0097] 3% HPMC aqueous solution appropriate amount
[0098] Prescription of gemesstat pellets:
[0099] jimmiesta 7
[0100] pregelatinized starch 4
[0101] Lactose 3
[0102] 3% HPMC aqueous solution appropriate amount
[0103] Prescription of Potassium Oxonate Potassium Pellets:
[0104] Potassium oxonate 18
[0105] pregelatinized starch 10
[0106] Lactose 6
[0107] 3% HPMC aqueous solution appropriate amount
[0108] other:
[0109] Sodium Lauryl Sulfate 16
[0110]
[0111] A total of 1000 capsules were made
[0112] The preparation process is the same as in Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com