Novel voriconazole broad-spectrum antifungal medicine compound, broad-spectrum antifungal medicine composition and application thereof
A technology of compounds and compositions, applied in antifungal agents, organic chemistry, pharmaceutical formulations, etc., can solve problems such as unclear mechanism of action, achieve strong antifungal activity, good safety, good pharmacokinetic properties and safety Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
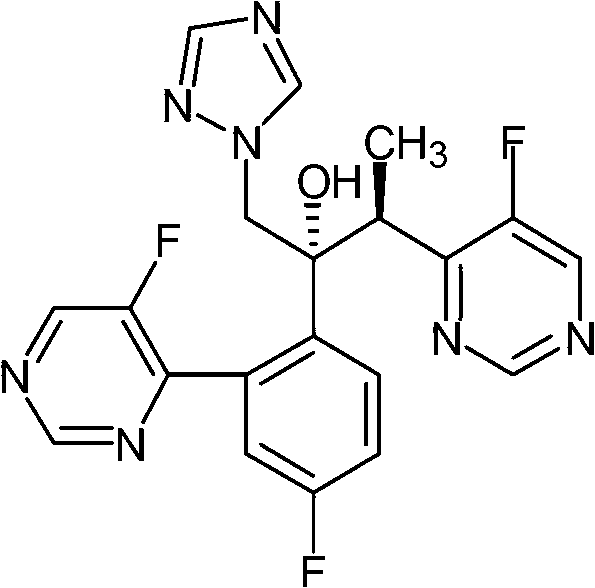
[0053] Preparation and identification test of embodiment 1, formula 1 compound
[0054] Step 1, add 2-chloro-2,4-difluoroacetophenone (27.3g, 0.14mol), 3,5 dibromo-1H-[1,2,4]triazole ( 34g, 0.15mol), potassium carbonate (27.6g, 0.2mol), tetrabutylammonium bromide (2.0g) and 300ml of dichloromethane, and stirred at reflux for 8 hours. Cool, filter, filter residue with CH 2 Cl 2 Wash, combine the organic layers, wash with water, dry, concentrate to dryness, and recrystallize with petroleum ether / ethyl acetate (1 / 1, 200ml) to obtain 35.0 g of a light yellow solid, which is directly used in the next step without purification .
[0055] Step 2, (2R,3S / 2S,3R)-3-(4-chloro-5-fluoropyrimidin-6-yl)-2-(2,4-difluorophenyl)-1-(3,5- Synthesis of Dibromo-1H-[1,2,4]-triazol-1-yl)butan-2-ol
[0056] Under nitrogen, the ethane solution (80ml, 0.2mol) of 2.5M n-butyllithium was added in the three-necked flask, and the tetrahydrofuran 100ml solution of diisopropylamine (20g, 0.2mol) was add...
Embodiment 2
[0061] Embodiment 2, preparation of the injection comprising the compound of formula 1
[0062] With the compound of formula 1 as the active ingredient, the injection is prepared according to the following formula and preparation process:
[0063] 2.1 Specifications: Each bottle contains 0.1g of new voriconazole.
[0064] 2.2 Composition and formula of injection:
[0065] Neovoriconazole (compound of formula 1) 100g
[0066] Ethanol 4L
[0067] Water for injection 20L
[0068] Made 1000 pieces
[0069] 2.3 Preparation process of injection:
[0070] In the aseptic operation room, weigh 100 g of the new voriconazole raw material (compound of formula 1), add 4 L of ethanol, stir to dissolve, add 30 g of activated carbon, stir for 30 minutes, filter and decarbonize, pass the filtrate through a 0.22 μm sterile membrane, Enter the 100-level clean tank; slowly add 20L of water for injection (refrigerated in the refrigerator) to the above solution, stir while adding, continue ...
Embodiment 3
[0071] Embodiment 3, comparative test of the adverse reaction rate of the compound of formula 1 and the original voriconazole compound
[0072] Under two different modes of administration (A oral administration / B intravenous infusion), 391 patients with acute invasive aspergillosis were given the new voriconazole compound (200 examples) shown in formula 1 of the present invention and the original voriconazole compound respectively. Product (original voriconazole compound, 191 cases) treatment, followed by other approved antifungal drug treatment, and evaluated the adverse reactions of formula 1 compound (180 cases) and original voriconazole (196 cases) in the treatment of esophageal candidiasis . The laboratory treatment effects of the compound of formula 1 and the original voriconazole compound are basically close, but the adverse reactions are greatly different. The specific comparison experiment results are shown in the following table:
[0073] Table 3. Comparative tes...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com