Cefalexin liposome and medicinal composition thereof
A technology of cephalexin and cephalexin, which is applied in the field of pharmaceutical compositions containing the cephalexin liposome, can solve the problems of poor stability, bioavailability, and low encapsulation efficiency, and achieve convenient clinical medication and low toxicity and side effects Small, full pharmacodynamic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
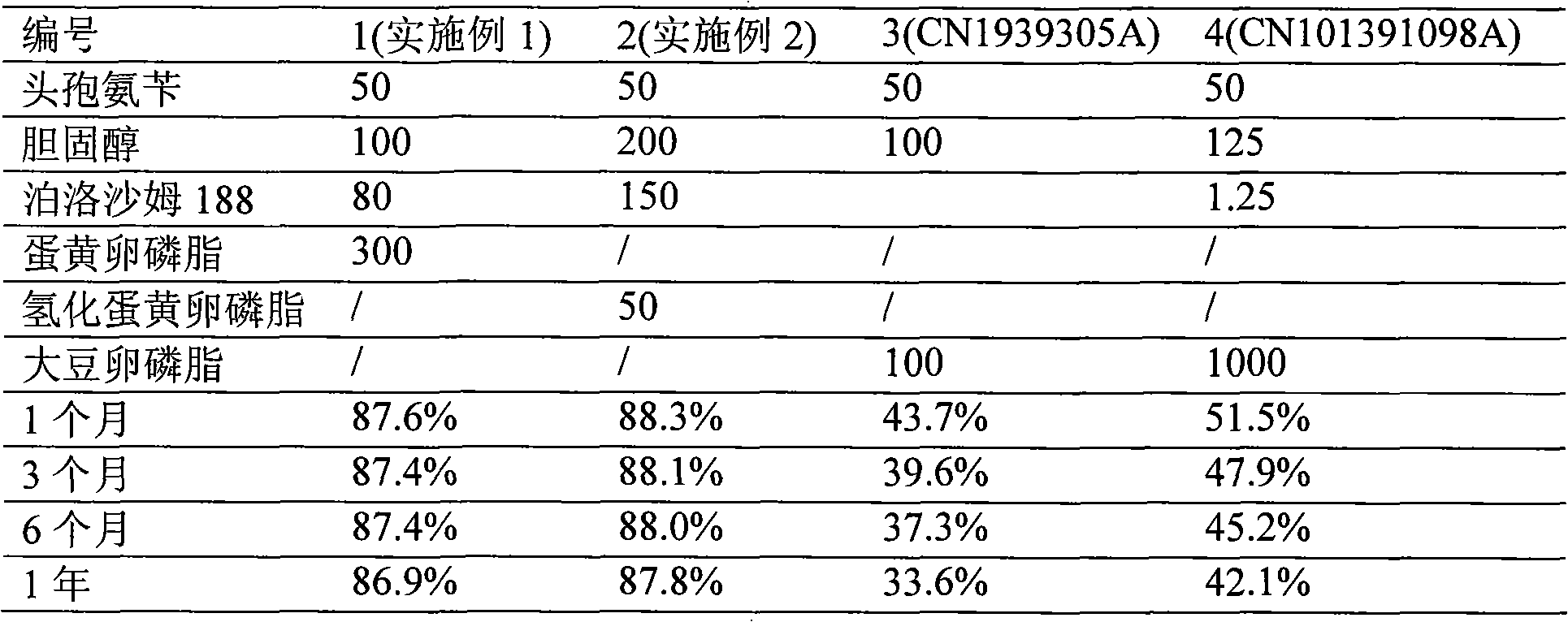
[0041] The preparation of embodiment 1 cephalexin liposome
[0044] Cholesterol 100g
[0045] Poloxamer 188 80g
[0046] Preparation Process
[0047] (1) Dissolve 50g cephalexin, 300g egg yolk lecithin, 100g cholesterol and 80g poloxamer 188 in 1500ml isopropanol, mix well, remove isopropanol under reduced pressure on a rotary thin film evaporator, and obtain a phospholipid film ;
[0048] (2) Add 1000ml of pH=6.0 carbonate buffer solution, shake and stir to make the phospholipid film fully hydrated, high-speed homogeneous emulsification, and obtain a liposome suspension;
[0049] (3) freeze-drying the suspension to prepare cephalexin liposomes.
Embodiment 2
[0050] The preparation of embodiment 2 cephalexin liposome
[0051] Prescription: Cefalexin 50g
[0052] Hydrogenated egg yolk lecithin 50g
[0053] Cholesterol 200g
[0054] Poloxamer 188 150g
[0055] Preparation Process
[0056] (1) Dissolve 50g cephalexin, 50g soybean lecithin, 200g cholesterol and 150g poloxamer 188 in 1500ml tert-butanol, mix well, remove tert-butanol under reduced pressure on a rotary thin film evaporator, and obtain a phospholipid film ;
[0057] (2) Add 1000ml of pH=6.0 sodium citrate buffer solution, shake, stir to make the phospholipid film fully hydrated, high-speed homogeneous emulsification, and obtain a liposome suspension;
[0058] (3) The suspension is spray-dried to obtain cephalexin liposomes.
Embodiment 3
[0059] The preparation of embodiment 3 cephalexin granules
[0060] Cefalexin liposome (calculated as cephalexin) 50g
[0061] Lactose 80g
[0062] Mannitol 160g
[0063] Dextrin 50g
[0064] Hypromellose 3g
[0065] Sucrose 120g
[0066] Chocolate essence 6g
[0067] Remarks: the meaning of "cephalexin liposome (calculated as cephalexin) 50g" is that the cephalexin liposome actually used contains 50g of cephalexin active ingredient, and the following examples are all explanations for this meaning.
[0068] Preparation Process
[0069] (1) Take 3g hypromellose and dissolve it in 150ml of 50% ethanol solution to make 2% hypromellose 50% ethanol solution, as a binding agent, for subsequent use;
[0070] (2) pulverize the liposome containing 50g cephalexin, cross 80 mesh sieves, and set aside;
[0071] (3) Weigh 80g lactose, 160g mannitol, 50g dextrin and 120g sucrose, pass through an 80 mesh sieve, mix, and set aside;
[0072] (4) Mix the above raw and auxiliary materia...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com