Full-implanted artificial corneal stroma and preparation method thereof
An artificial cornea, implantable technology, applied in the field of biomedical engineering, to achieve the effect of sufficient sources, wide distribution, avoiding toxic reactions and inducing rejection reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
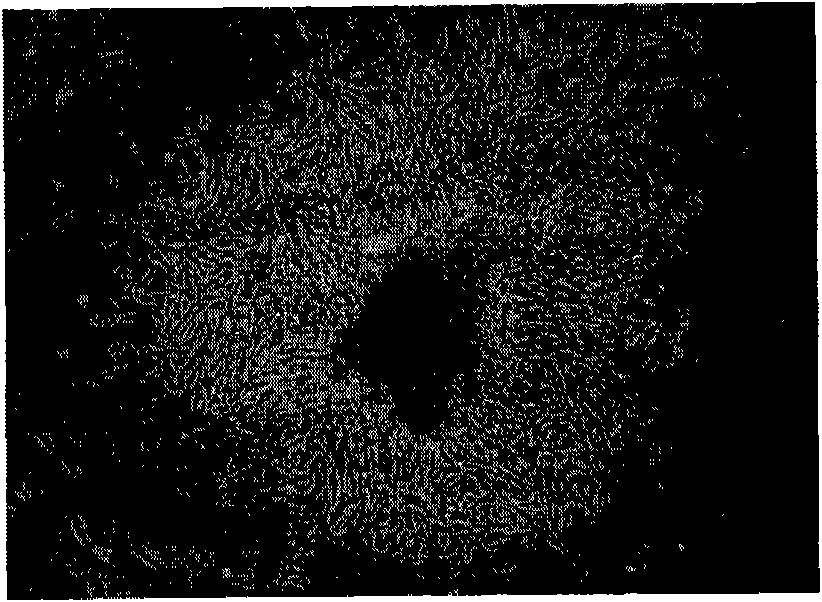
Image
Examples
Embodiment 1
[0050] Example 1 In vitro preparation and detection of fully implantable artificial corneal stroma
[0051] 1. Experimental apparatus and reagents 6-month-old female New Zealand white rabbits, weighing 2-2.5 kg (Shanghai Animal Research Center of Chinese Academy of Sciences). DMEM (Dulbeco'smodified eagle'smedium) powder is the product of GIBCO Company in the United States; collagenase type I is the product of Worthington Company in the United States; fetal bovine serum (Fetal Bovine Serum, FBS) is the product of Hyclone Company in the United States; dexamethasone and transferrin are The product of Sigma Company in the United States; L-glutamine, ascorbic acid and trypsin are products of Shanghai Shisheng Biotechnology Co., Ltd.; penicillin and streptomycin are products of North China Pharmaceutical Co., Ltd.; NaHCO 3 It is a product of Shanghai Hongguang Chemical Factory. LSM-510 two-photon microscope was purchased from Zeiss, Germany. JSM-6701F scanning electron microscope...
Embodiment 2
[0061] The in vivo (animal) experiment of embodiment 2 fully implanted artificial corneal stroma
[0062] 1. Experimental apparatus 6-month-old female New Zealand white rabbits, weighing 2-2.5 kg (Shanghai Animal Research Center of Chinese Academy of Sciences). DMEM (Dulbeco's modified eagle's medium) powder is a product of GIBCO Company of the United States; collagenase type I is a product of Worthington Company of the United States; fetal bovine serum (Fetal Bovine Serum, FBS) is a product of Hyclone Company of the United States; dexamethasone and transferrin are products of Sigma Corporation of the United States. The company's products; L-glutamine, ascorbic acid and trypsin are products of Shanghai Shisheng Biotechnology Co., Ltd.; penicillin and streptomycin are products of North China Pharmaceutical Co., Ltd.; NaHCO 3 It is a product of Shanghai Hongguang Chemical Factory; vimentin monoclonal antibody was purchased from American Lab Vision; smooth muscle protein monoclon...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com