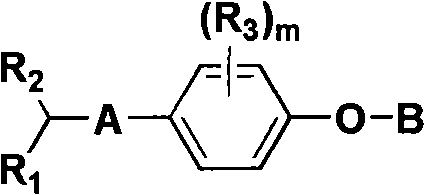
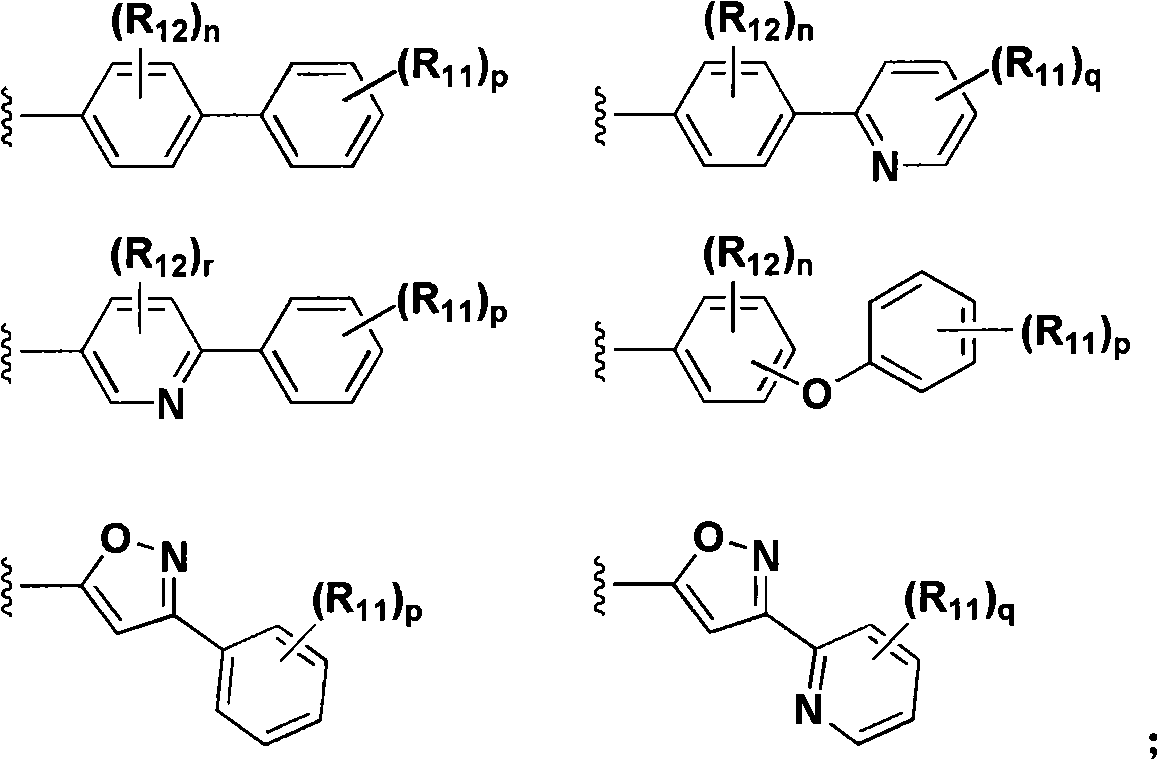
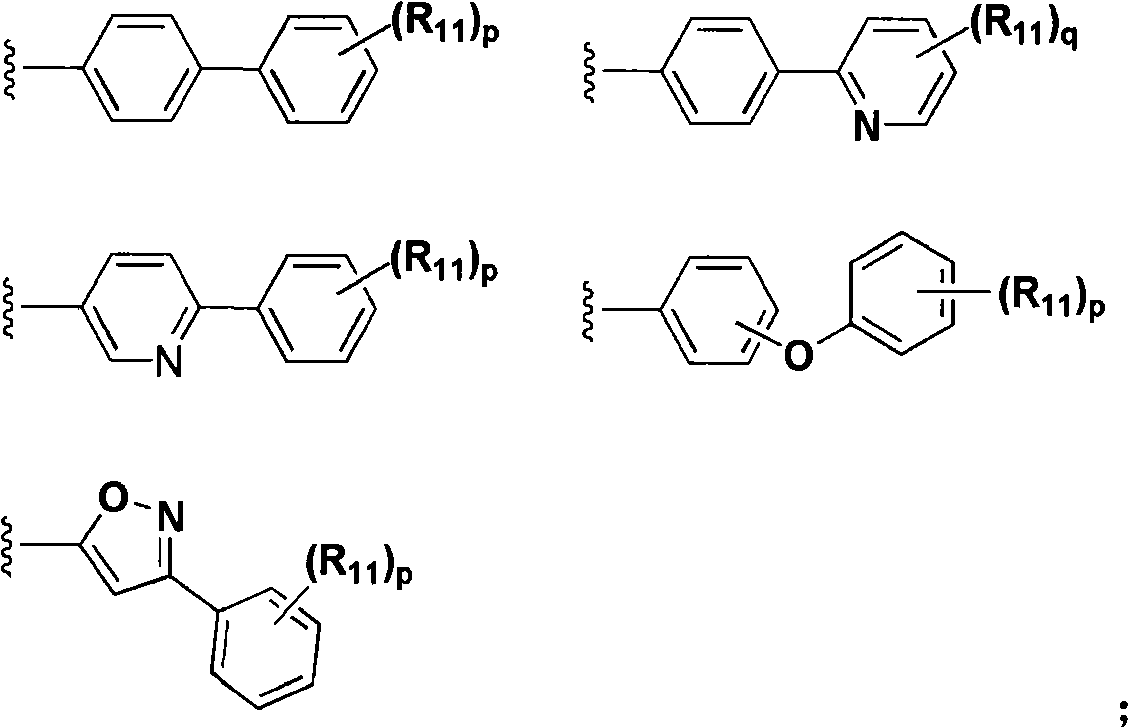
Aryl compounds as ppar ligands and their use
A kind of compound, the technology of ester compound, be used in the aryl compound as PPAR ligand and its use field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Embodiment 1: preparation compound S1
[0098] [Process A]
[0099] 468 mg (2 mmol) of 4-iodo-2-methylphenol were dissolved in 20 ml of anhydrous tetrahydrofuran in the presence of nitrogen while maintaining the temperature at 0°C. 1.5 ml of isopropylmagnesium chloride (2M) was slowly added thereto, followed by reaction for 10 minutes. The reaction solution was cooled to -78°C, and 2.00 ml of tert-butyllithium (1.7M hexane solution, 1.0 equivalent) was slowly added thereto. After stirring for 10 minutes, 64 mg (2 mmol, 1.0 equivalent) of solid S (sulfur) was added thereto at the same temperature in one portion. The reaction was continued for 40 minutes at an elevated temperature of 15°C. Dissolve 541 mg (2 mmol, 1.0 equivalent) of 4-chloromethyl-4'-trifluoromethyl-biphenyl of formula (III) in 10 ml of anhydrous THF, and slowly add it to the above reactant at the same temperature middle. After reacting for another hour, ammonium chloride solution was used to termi...
Embodiment 2
[0101] Embodiment 2: preparation compound S2
[0102] [Process B]
[0103] 748 mg (2 mmol) of compound S1 and 290 mg (2.0 equivalents) of imidazole were completely dissolved in 20 ml of dimethylformamide. 165 mg (1.1 equivalents) of tert-butyldimethylchlorosilane was slowly added thereto, followed by stirring at room temperature for 4 hours. After the reaction was completed, the organic solvent was extracted using ammonium chloride solution and ethyl acetate. Moisture in the organic layer was dried using magnesium sulfate. Purification was performed using a silica gel column, and the solvent was distilled off under reduced pressure to obtain 928 mg (yield: 95%) of the target compound.
[0104] 1 H NMR (300MHz, CDCl 3 )δ7.67(s, 4H), δ7.50(d, 2H), δ7.27(t, 2H), δ7.13(s, 1H), δ7.05(q, 1H), δ6.66( d, 1H), δ4.04(s, 2H), δ2.15(s, 3H), δ1.01(s, 9H), δ0.20(s, 6H).
Embodiment 3
[0105] Embodiment 3: preparation compound S3
[0106] [Process C]
[0107] 977 mg (2 mmol) of compound S2 was dissolved in 20 ml of anhydrous tetrahydrofuran, and the temperature was lowered to -78°C. 3.6 ml (1.8 M, 2.0 equivalents) of lithium diisopropylamide (LDA) was slowly added thereto. Then, 274 µl (2.0 mmol) of benzyl bromide was added to the reaction solution, and the temperature was slowly raised to room temperature. After reacting for another 30 minutes, ammonium chloride solution was used to terminate the reaction, ethyl acetate and sodium chloride solution were used to extract the organic solvent, and magnesium sulfate was used to dry to remove moisture in the organic layer. After filtration, the solvent was distilled off under reduced pressure, and the residue was further treated by silica gel column chromatography to obtain 961 mg (yield: 83%) of the target compound.
[0108] 1 H NMR (300MHz, CDCl 3 )δ7.67(s, 4H), δ7.47~7.05(m, 11H), δ6.63(d, 1H), δ4.30(m,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com