Selective glycosidase inhibitors and uses thereof
An optional, compound technology, applied in the field of compounds that selectively inhibit glycosidase, can solve the problems of lack of selectivity, interference with multiple cellular processes, unfavorable use, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
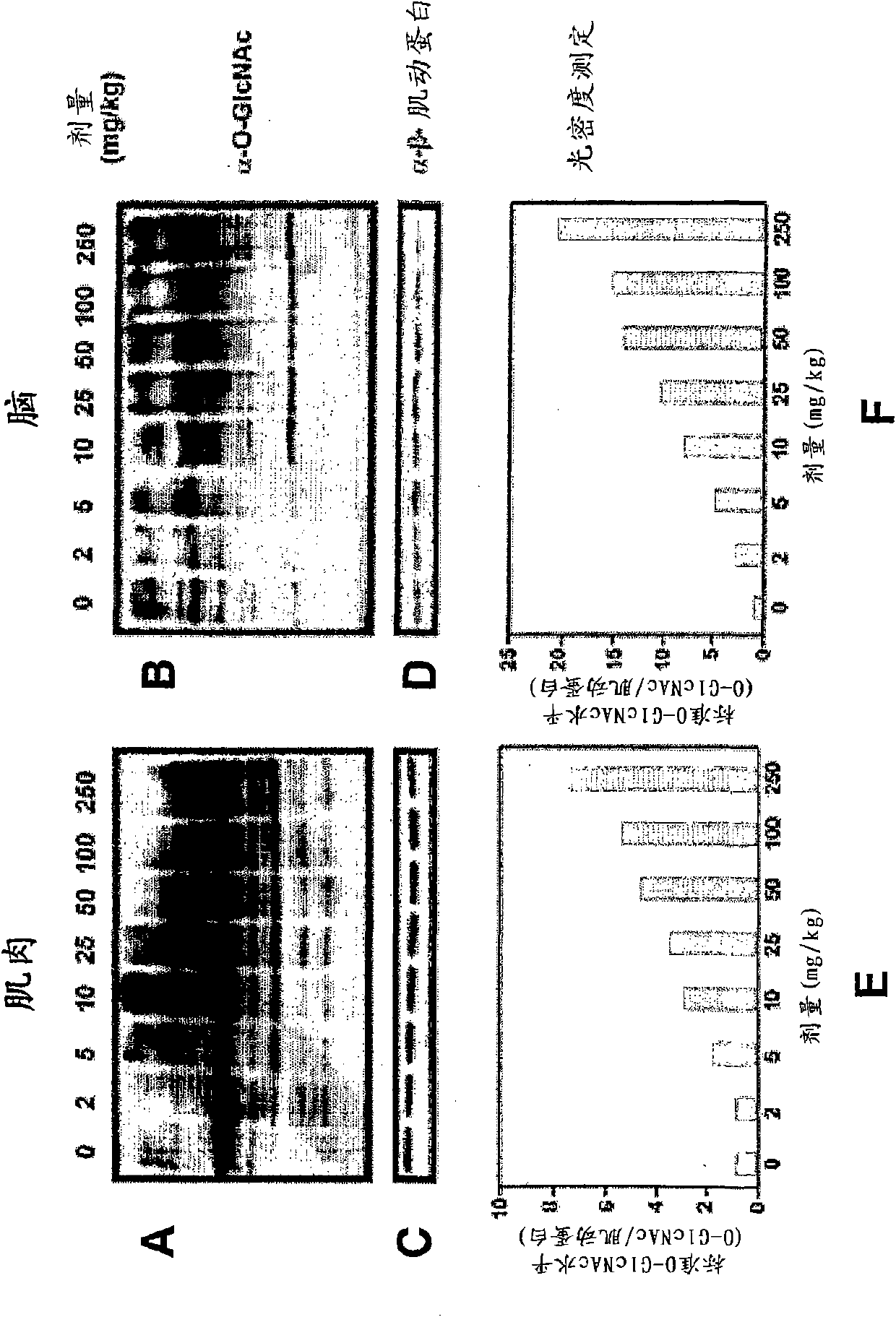
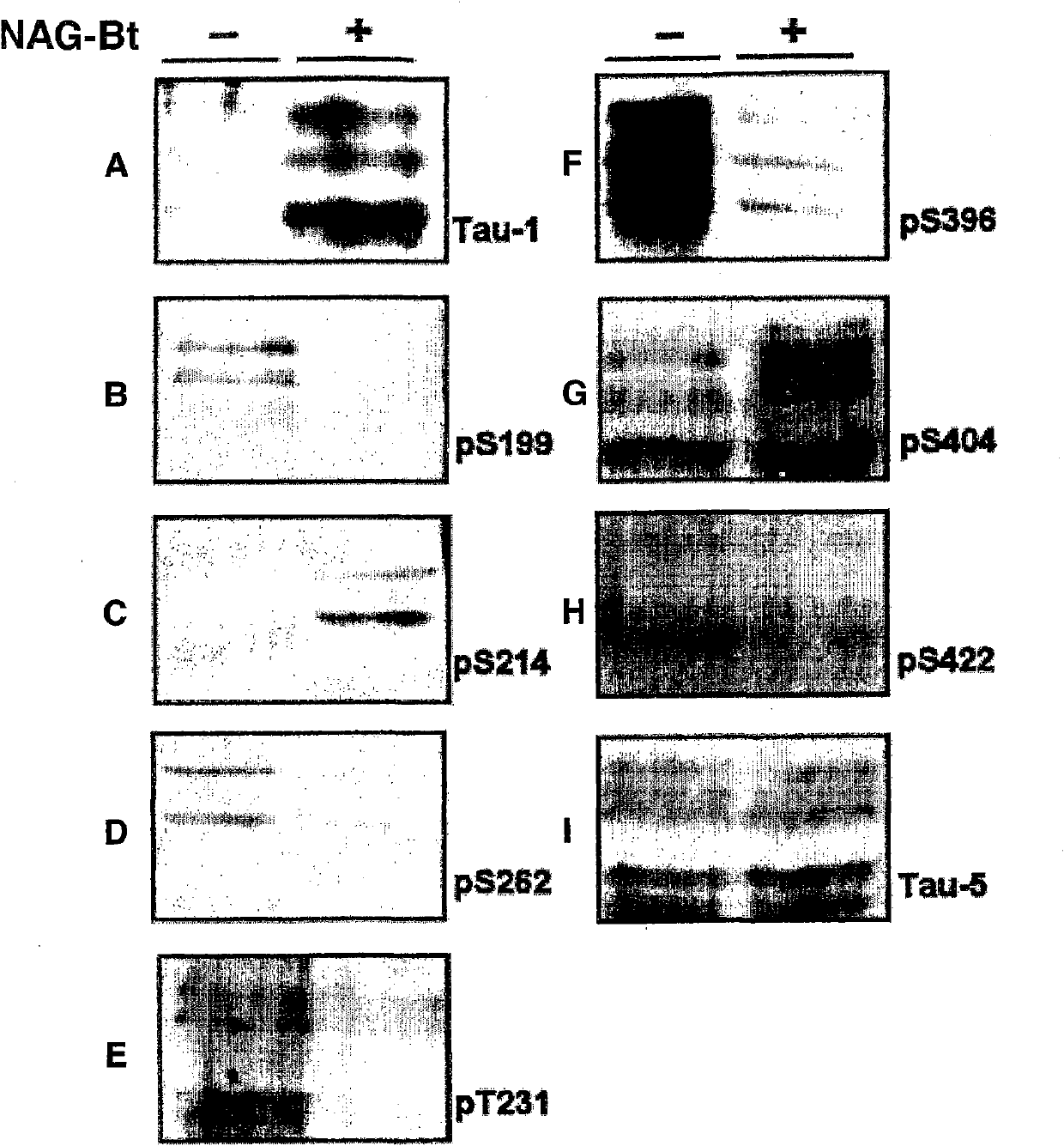
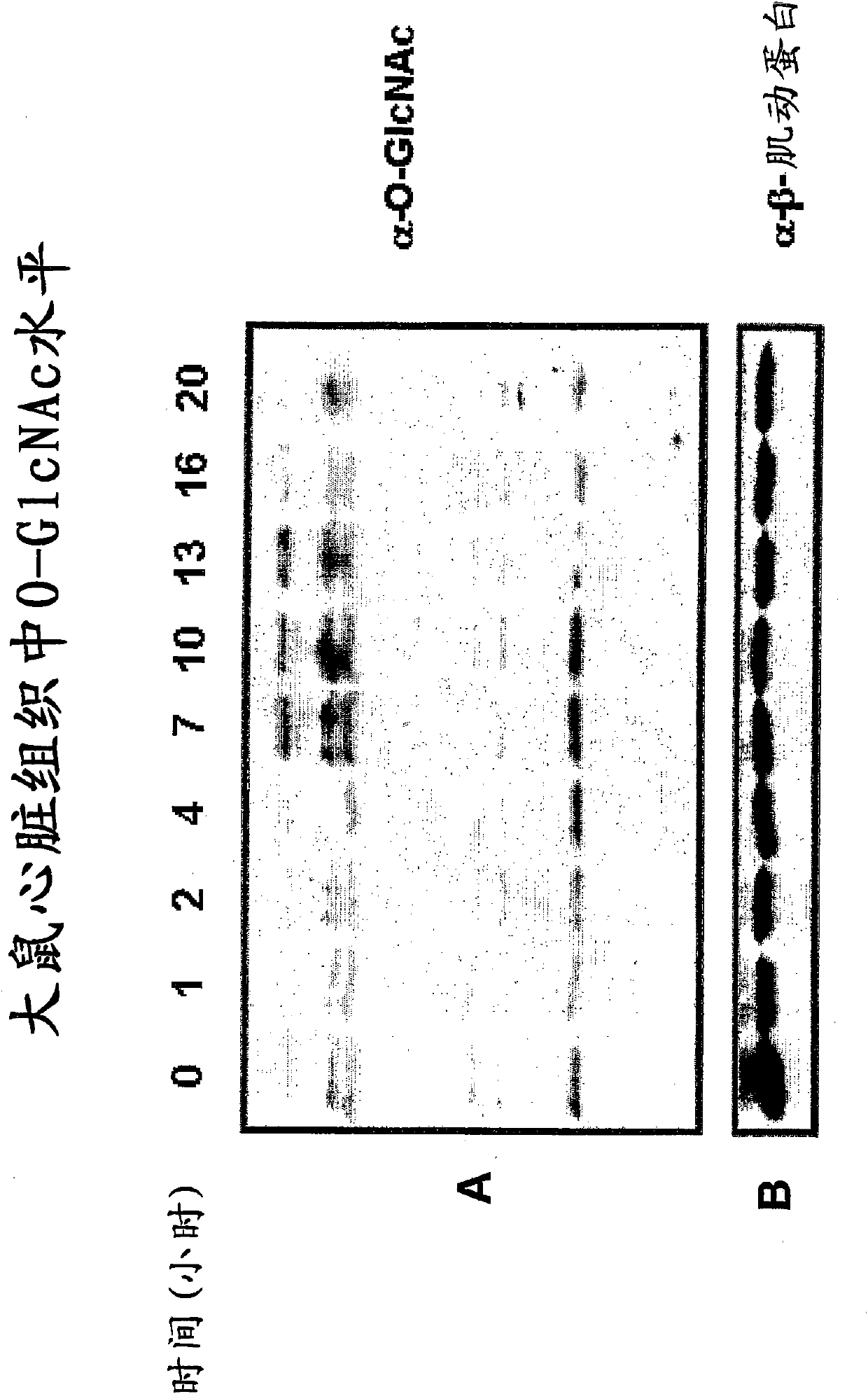
Image
Examples
Embodiment 1
[0194] Compounds 1 and 2: diacetic acid (3aR, 5R, 6S, 7R, 7aR)-5-(acetoxymethyl)-2-(fluoromethyl)- 5,6,7,7a-tetrahydro-3aH-pyrano[3,2-d]thiazole-6,7-diyl ester (1) and (3aR, 5R, 6S, 7R, 7aR)-2-(fluoromethyl)-5-(hydroxymethyl)-5,6,7,7a-tetrahydro-3aH-pyrano [3,2-d]thiazole-6,7-diol (2)
[0195]
[0196] Add triethylamine (0.8 mL) and anhydrous pyridine (20 mL) to 2-amino-2-deoxy-1,3,4,6 tetra-O-acetyl-β-D-glucopyranose hydrochloride (1 g ) in a cold (0° C.) solution in DMF (100 mL). Sodium fluoroacetate (1.8 g) was added to a stirred solution containing dry Dowex 50-H + Resin (12g) in anhydrous DMF (90mL) mixture. After 1 hour, DCC (3.2 g) and 30 mL of fluoroacetic acid solution were added via cannula to the reaction vessel containing the hydrochloride salt. The resulting solution was allowed to stand at 0°C for 16 hours, after which time the reaction was judged complete by TLC analysis. Part of the solvent was removed in vacuo, EtOAc (300 mL) and saturated sodium...
Embodiment 2
[0200] Compounds 3 and 4: diacetic acid (3aR, 5R, 6S, 7R, 7aR)-5-(acetoxymethyl)-2-(difluoromethane base)-5,6,7,7a-tetrahydro-3aH-pyrano[3,2-d]thiazole-6,7-diyl ester (3) and (3aR, 5R, 6S, 7R, 7aR)-2-(difluoromethyl)-5-(hydroxymethyl)-5,6,7,7a-tetrahydro-3aH-pyran And[3,2-d]thiazole-6,7-diol (4)
[0201]
[0202]Add triethylamine (0.8 mL) and anhydrous pyridine (20 mL) to 2-amino-2-deoxy-1,3,4,6 tetra-O-acetyl-β-D-glucopyranose hydrochloride (1 g ) in a cold (0° C.) solution in DMF (100 mL). Dicyclohexylcarbodiimide (DCC, 3 g) and difluoroacetic acid (1.2 mL) were added to the reaction mixture via syringe. The resulting solution was allowed to stand at 0° C. for 16 hours, after which 0.5 mL of difluoroacetic acid was added. After standing at room temperature for another 3.5 hours, the reaction was judged to be complete by TLC analysis. Part of the solvent was removed in vacuo, EtOAc (300 mL) and saturated sodium chloride solution (100 mL) were added. The organic ...
Embodiment 3
[0206] Compounds 5 and 6: diacetic acid (3aR, 5R, 6S, 7R, 7aR)-5-(acetoxymethyl)-2-(trifluoromethane base)-5,6,7,7a-tetrahydro-3aH-pyrano[3,2-d]thiazole-6,7-diyl ester (5) and (3aR, 5R, 6S, 7R, 7aR)-5-(hydroxymethyl)-2-(trifluoromethyl)-5,6,7,7a-tetrahydro-3aH-pyran And[3,2-d]thiazole-6,7-diol (6)
[0207]
[0208] Add triethylamine (0.8 mL) to 2-amino-2-deoxy-1,3,4,6-tetra-O-acetyl-β-D-pyridine dissolved in anhydrous dichloromethane (20 mL) In a cold (0°C) solution of glucopyranose hydrochloride (1g). Trifluoroacetic anhydride (0.6 mL) was added via syringe and the resulting solution was allowed to stand at 0°C for 16 hours after which time the reaction was judged complete by TLC analysis. The solution was diluted in 50mL EtOAc and washed successively with water, saturated NaHCO 3 aqueous solution (2x) and finally brine solution. with MgSO 4 The organic extracts were dried and filtered, and the solvent was removed in vacuo to give a colorless syrup. The desired...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com