Novel method for preparing S-pantoprazole and salt
A pantoprazole, S-1 technology, applied in the field of medicine, can solve problems such as difficult to meet, high price, complicated preparation process of chiral reagents, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
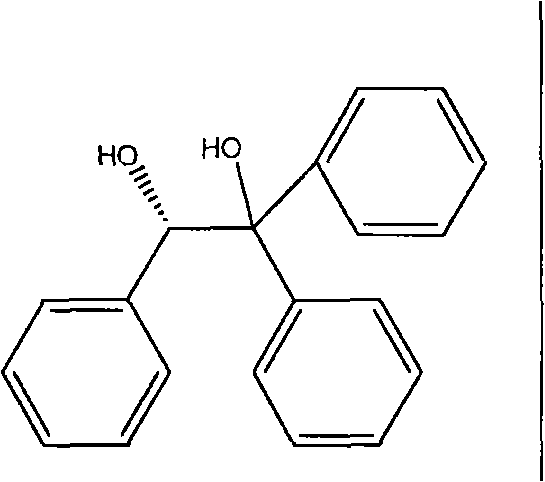
[0073] Embodiment 1 prepares S-pantoprazole and S-1,1,2-triphenyl-1,2-ethylene glycol compound
[0074] Referring to the method described in WO2007 / 074099, the chiral resolution reagent S-1,1,2-triphenyl-1,2-ethylene glycol is reacted with racemic pantoprazole in a solvent, and cooled after the reaction is completed To the crystallization temperature, filter the S-pantoprazole and S-1,1,2-triphenyl-1, the compound precipitation of 2-ethanediol that generate after crystallization is finished, use 2 times of (V / W) The filter cake was washed with solvent and dried under vacuum at 40°C. The specific operating conditions and results are shown in Table 1.
[0075] Table 1 prepares the operating conditions and results of S pantoprazole and S-1,1,2-triphenyl 1,2-ethylene glycol complex
[0076] solvent
Dosage
racemic pantoprazole
Resolution reagent
opposite
answer
warm...
Embodiment 2
[0080] The recrystallization of embodiment 2 complex
[0081] The complex with ee value of 92% was mixed with a recrystallization solvent, heated to dissolve, cooled to crystallize, and filtered to obtain the product. See Table 2 for specific operating conditions.
[0082] The specific operating conditions and results of the complex recrystallization in table 2
[0083] solvent
Dosage
composite
thing
crystallization temperature
Spend
when crystallized
between
Product yield
yield
ee value
methyl isobutyl
base ketone
25ml
1g
70℃
15℃
8h
0.89g
89.0%
97.9%
Alkane=84:
16
25ml
1g
70℃
15℃
8h
0.73g
73%
97.3%
25ml
1g
70℃
15℃
8h
...
Embodiment 4
[0089] Embodiment 4 uses dichloromethane to carry out the recrystallization of complex
[0090] The complex prepared in Example 3 was mixed with dichloromethane, heated to dissolve, cooled to crystallize, filtered, and vacuum-dried at 35°C to obtain the product. See Table 4 for specific operating conditions.
[0091] Table 4 uses methylene chloride to carry out the specific operating conditions and results of complex recrystallization
[0092] Solvent consumption
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com