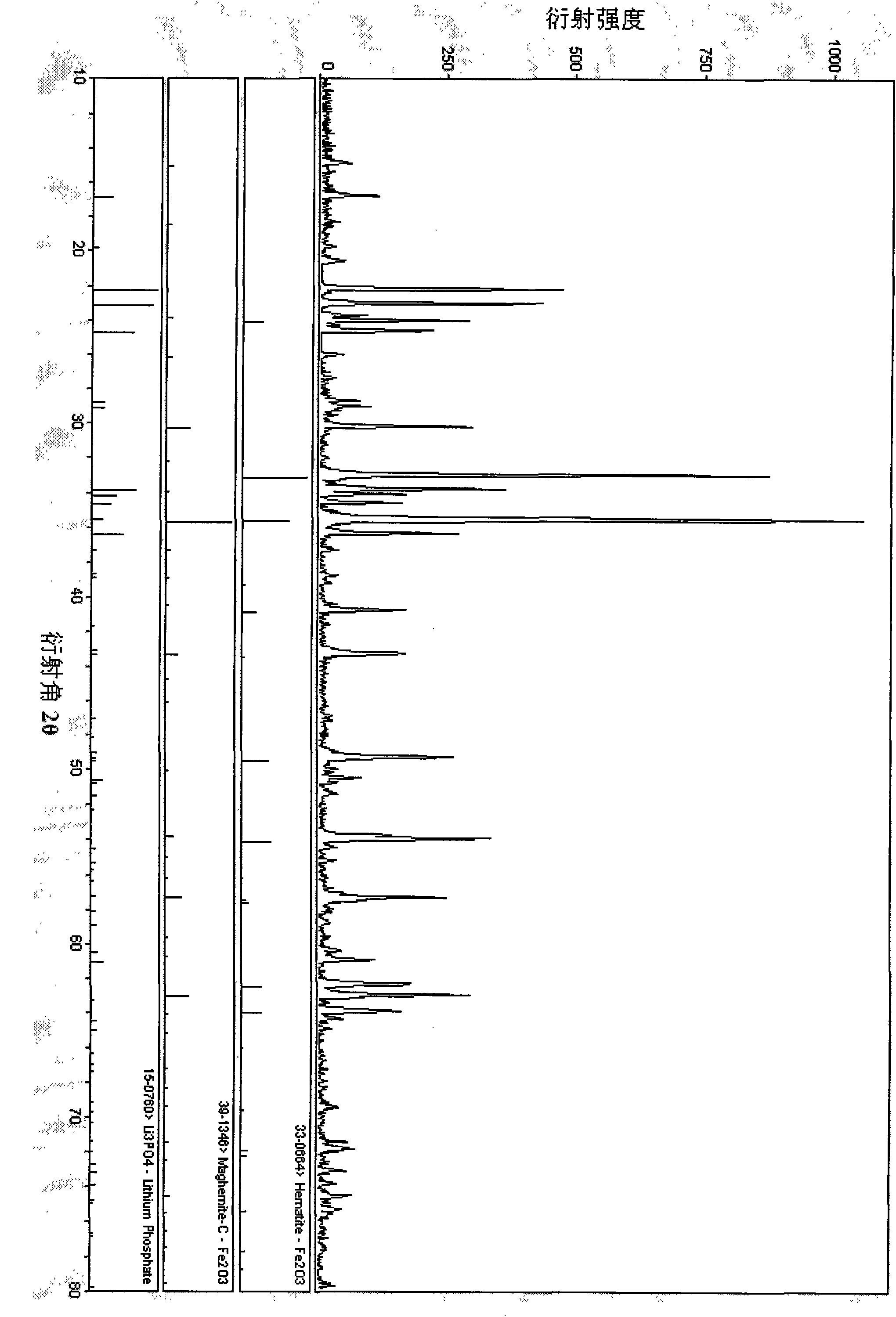
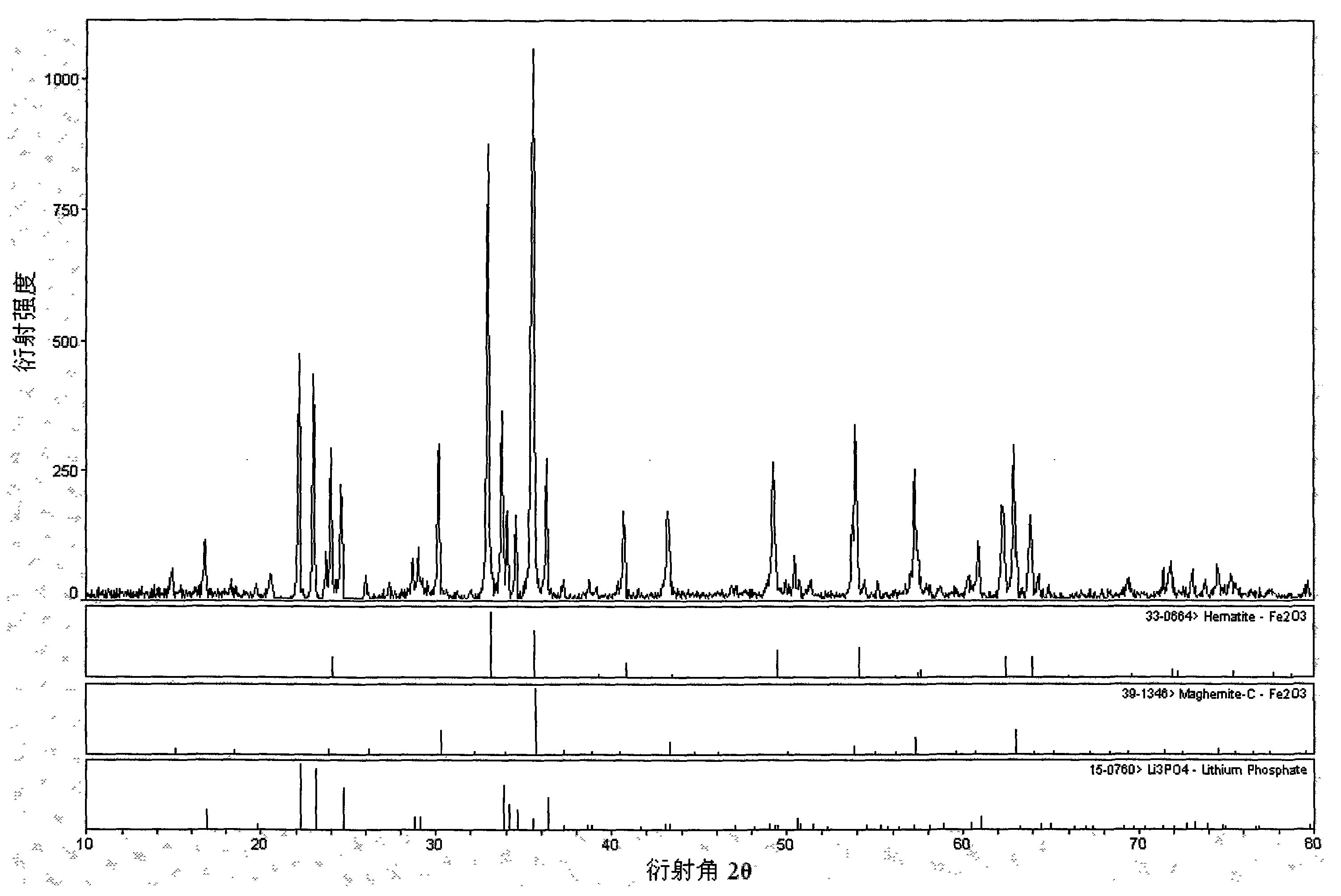
Method for preparing Li3PO4 and Fe2O3 by ferrophosphorus
A fe2o3, ferrophosphorus technology, applied in the field of materials, can solve the problems of reducing oxygen purity and oxygen accuracy, difficult oxygen control, equipment corrosion, etc., to achieve the effect of eliminating severe corrosion, shortening the utilization process, and lower requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Preparation of Li from FeP and LiOH by reactive pulverization method 3 PO 4 with Fe 2 o 3 , first crush ferrophosphorus to a certain particle size, use ferrophosphorus as the phosphorus source, and LiOH as the lithium source, the ratio of the two substances is 1:3, after ball milling for 5 hours, put it into a horse at 300-900°C Roasting in a Furnace for 0.5-10 hours, heating and cooling in the air, using oxygen in the air as an oxygen source, without controlling the amount of air, and without other protective atmospheres, to obtain Li 3 PO 4 with Fe 2 o 3 , the reaction equation is as follows:
[0022] 2FeP+6LiOH+4O 2 →2Li 3 PO 4 +Fe 2 o 3 +3H 2 o
Embodiment 2
[0024] Using the rheological phase method to obtain the by-product phosphorus iron Fe of the yellow phosphorus production plant 1.5 P as raw material to prepare Li 3 PO 4 with Fe 2 o 3 , first crush ferrophosphorus to a certain particle size with a ball mill, use ferrophosphorus as the phosphorus source, and use Li 2 CO 3 It is a lithium source, the ratio of the two substances is 2:3, and ethanol is used as a solvent. After being fully ball-milled or ground to form a rheological phase, it is put into a muffle furnace at 200-900°C for 1-25 hours and baked in the air. Heating and cooling, using oxygen in the air as an oxygen source, no need to control the amount of air, no other protective atmosphere, to get Li 3 PO 4 with Fe 2 o 3 , the reaction equation is as follows:
[0025] 8Fe 1.5 P+12Li 2 CO 3 +19O 2 →8Li 3 PO 4 +6Fe 2 o 3 +12CO 2
[0026] Among them, the by-product CO 2 Can be absorbed by LiOH solution to form Li 2 CO 3 Raw materials to achieve zero...
Embodiment 3
[0028] By-product Fe of calcium magnesium phosphate fertilizer production plant by spray drying method 2 P as raw material to prepare Li 3 PO 4 with Fe 2 o 3 , first crush ferrophosphorus to a certain particle size with a jet mill, use ferrophosphorus as the phosphorus source, and use LiH 2 PO 4 LiOH and LiOH are mixed lithium sources, and the ratio of the three substances is 1:1:5. After the precursor is atomized, it is placed in an oxygen atmosphere furnace at 200-900°C. It is not necessary to control the amount of oxygen, and it is not necessary to For other protective atmospheres, get Li 3 PO 4 with Fe 2 o 3 , the reaction equation is as follows:
[0029] 4Fe 2 P+4LiH 2 PO 4 +20LiOH+9O 2 →8Li 3 PO 4 +4Fe 2 o 3 +10H 2 o
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com