Bi(diallylalkylamine) and preparation method thereof
A technology of bis-diallylalkylamine and diallylamine, applied in the field of bis-diallylalkylamine and its preparation, can solve the problem of polymerization of bis-diallylalkylamine and its quaternary ammonium salt There are no literature disclosures in the research and development of biological substances, so as to achieve the effects of easy popularization and industrialization, environmental protection of the process, and reduction of manufacturing costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
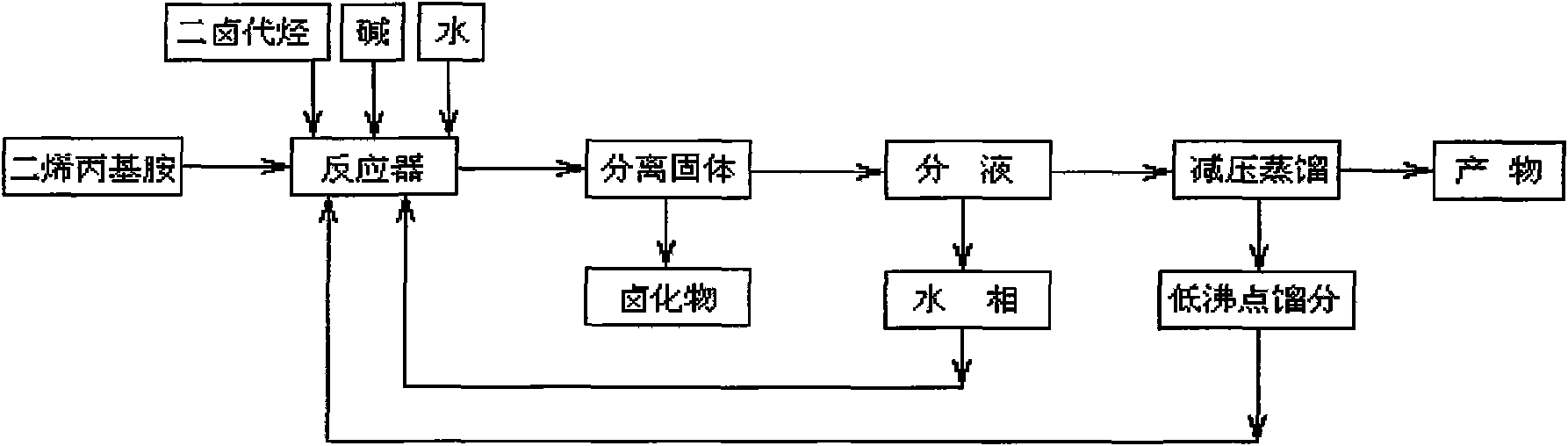
[0040] (1) According to the molar ratio of diallylamine, 1,2-dibromoethane and alkali at 2.0:1:2.0, 42.7mL of diallylamine, 15mL of 1,2-dibromoethane, 36.55 gNa 2 CO 3 Add 100mL of water into a 250mL three-necked bottle with a mechanical stirring and reflux device, stir well, react at 60°C for 24h, separate the solid to obtain the liquid phase;
[0041] (2) The above liquid phase is transferred to a 200mL separatory funnel, and after standing for 30min, the liquid is separated to obtain the oil phase;
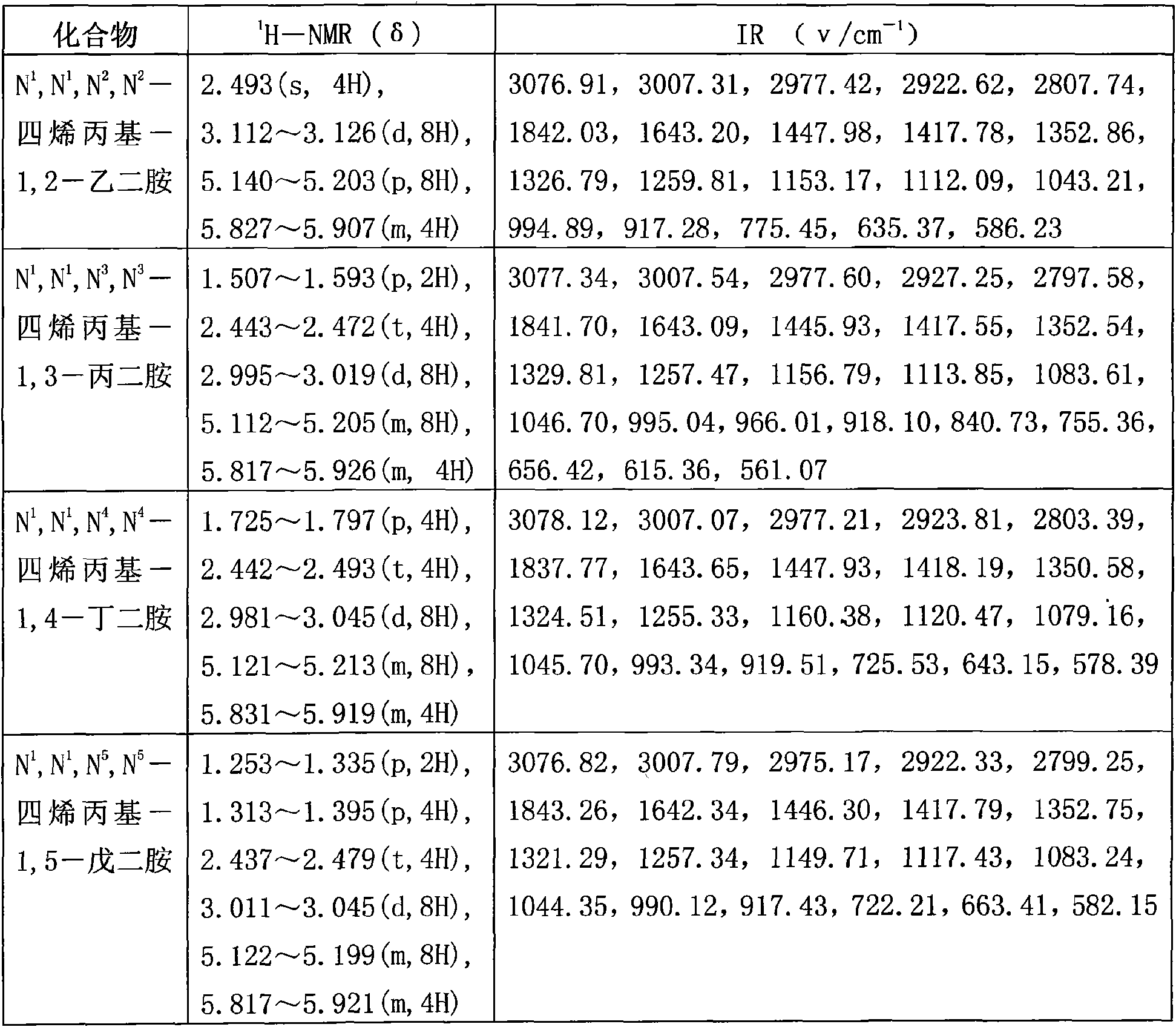
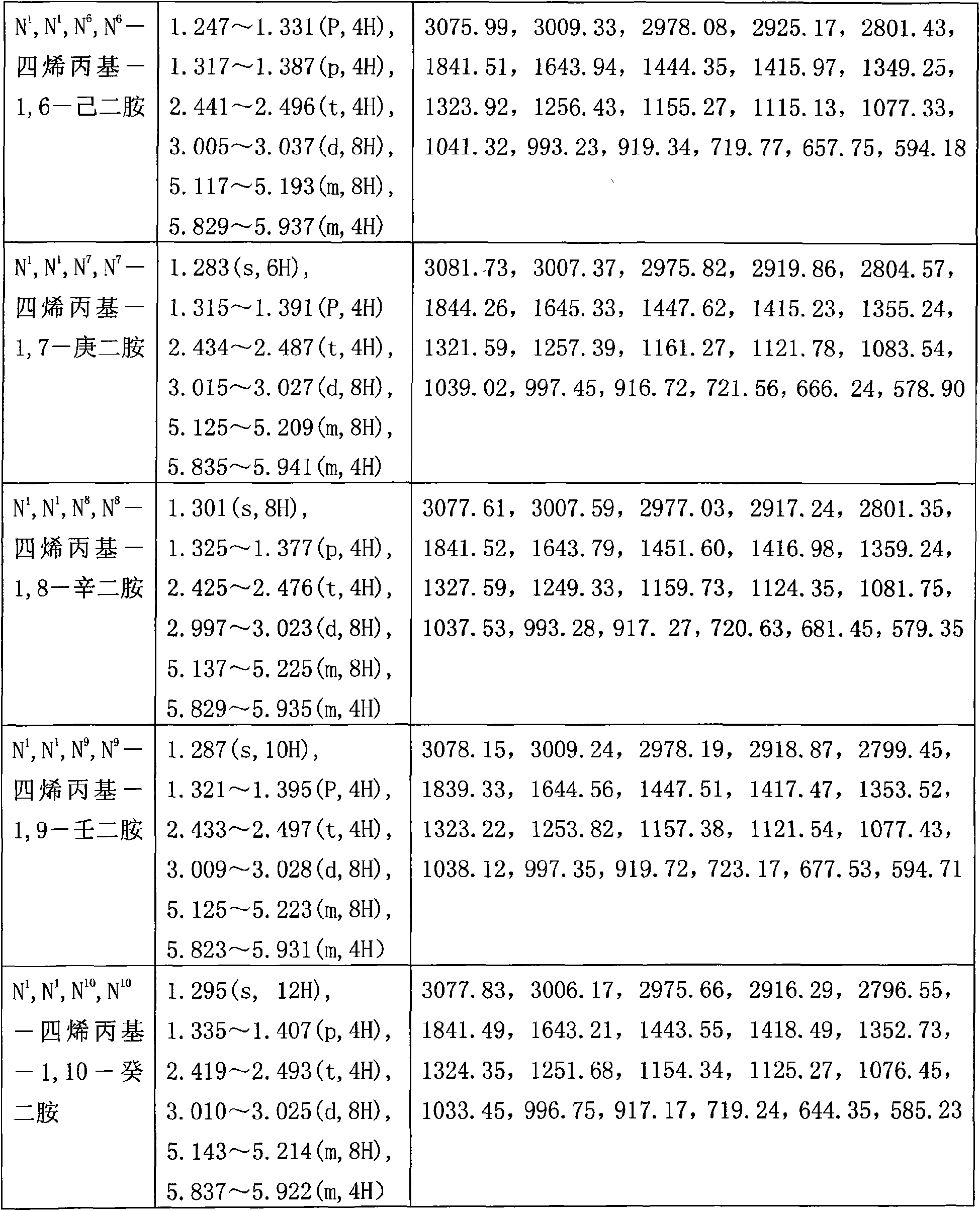
[0042] (3) Add 29.1mg of polymerization inhibitor hydroquinone to the above oil phase and carry out vacuum distillation, collect the fraction at 122~125°C / 10×133.3Pa to obtain 32.8mL of colorless transparent liquid, namely N 1 , N 1 , N 2 , N 2 -tetraallyl-1,2-ethylenediamine, the yield is 76.40%;
[0043] (4) collecting step (2) liquid separation gained aqueous phase and step (3) vacuum distillation gained low-boiling fraction;
[0044] (5) Collect the solid obtained in...
Embodiment 2
[0046] (1) The aqueous phase and the low boiling point fraction collected in step (4) in Example 1 are added in the three-necked flask and then participate in this round of reaction;
[0047] (2) According to the molar ratio of diallylamine, 1,2-dibromoethane and alkali as 2.4:1:2.1, 51.2mL of diallylamine, 15mL of 1,2-dibromoethane and 49.28 K 2 CO 3 Add it into a 250mL three-necked bottle with a mechanical stirring and reflux device, stir well, react at 80°C for 15h, separate the solid to obtain the liquid phase;
[0048] (3) The above liquid phase was transferred to a 200mL separatory funnel, and after standing for 30min, the liquid was separated to obtain the oil phase;
[0049] (4) Add 47.9 mg of polymerization inhibitor hydroquinone to the above oil phase and carry out vacuum distillation, collect the fraction at 122~125°C / 10×133.3Pa to obtain 36mL of colorless transparent liquid, namely N 1 , N 1 , N 2 , N 2 -tetraallyl-1,2-ethylenediamine, the yield is 83.86%;
...
Embodiment 3
[0053] (1) The aqueous phase and the low-boiling point fraction collected in step (5) in Example 2 are added in the three-necked flask and then participate in this round of reaction;
[0054] (2) According to the molar ratio of diallylamine, 1,2-dibromoethane and alkali as 2.6:1:2.2, 55.5mL diallylamine, 15mL1,2-dibromoethane and 51.62 K 2 CO 3 Add it into a 250mL three-necked bottle with a mechanical stirring and reflux device, stir well, react at 100°C for 12h, separate the solid to obtain the liquid phase;
[0055] (3) The above liquid phase was transferred to a 200mL separatory funnel, and after standing for 30min, the liquid was separated to obtain the oil phase;
[0056] (4) Add 64.1 mg of polymerization inhibitor hydroquinone to the above oil phase for vacuum distillation, collect fractions at 122~125°C / 10×133.3Pa, and obtain 36.1 mL of colorless transparent liquid, namely N 1 , N 1 , N 2 , N 2 -tetraallyl-1,2-ethylenediamine, the yield is 84.09%;
[0057] (5) co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Intrinsic viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com