Preparation method of SnS2 nanoplate anode material of a lithium-ion battery
A technology of lithium-ion batteries and negative electrode materials, which is applied in the direction of electrode manufacturing, battery electrodes, circuits, etc., to achieve the effect of good cycle stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] 1) Dissolve 0.98g (8mmol) L-cysteine in 160ml deionized water, then add 0.70g (2mmol) tin tetrachloride (SnCl 4 ·5H 2 O), and stirred to make it dissolve, L-cysteine and SnCl in the mixed solution 4 The molar ratio is 4:1.
[0019] 2) The obtained mixed solution was transferred to a polytetrafluoroethylene liner reactor, sealed, and the reactor was kept at 180° C. for 8 hours, and then naturally cooled to room temperature. The precipitate was separated by centrifugation, washed thoroughly with deionized water and absolute ethanol, and dried in vacuum to obtain the SnS 2 Nanosheet anode materials.
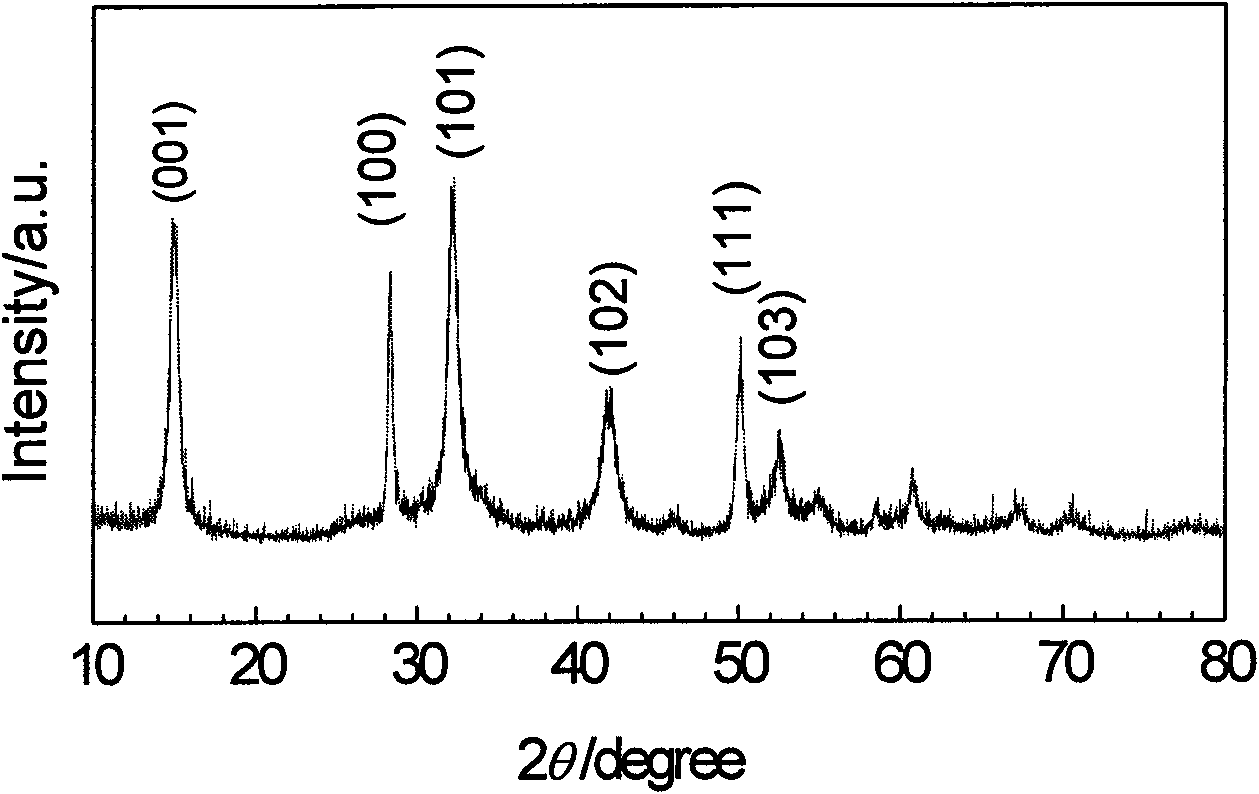
[0020] X-ray diffraction (XRD) analysis and transmission electron microscope (TEM) observation results show that the obtained product is SnS 2 Nanosheets (see figure 1 and figure 2 ).
[0021] 3) Electrochemical performance test: an appropriate amount of SnS 2 The nano sheet negative electrode material, the conductive agent acetylene black, and 5% of the binder p...
Embodiment 2
[0023] 1) Dissolve 1.45g (12mmol) L-cysteine in 150ml deionized water, then add 0.7g (2mmol) tin tetrachloride (SnCl 4 ·5H 2 O), and stirred to make it dissolve, L-cysteine and SnCl in the mixed solution 4 The molar ratio is 6:1.
[0024] 2) The obtained mixed solution was transferred to a polytetrafluoroethylene liner reactor, sealed, and the reactor was kept at 190°C for 12 hours, and then naturally cooled to room temperature. The precipitate was separated by centrifugation, washed thoroughly with deionized water and absolute ethanol, and dried in vacuum to obtain the SnS 2 Nanosheet anode materials. X-ray diffraction (XRD) analysis and transmission electron microscope (TEM) observation results show that the obtained product is SnS 2 Nanosheets (see image 3 )
[0025] 3) by the 3rd of embodiment 1) the method for step is assembled into test cell, and by the 3rd of embodiment 1) the test method test SnS of step 2 Electrochemical lithium storage performance of nano...
Embodiment 3
[0027] 1) Dissolve 1.45g (12mmol) L-cysteine in 150ml deionized water, then add 0.52g (1.5mmol) tin tetrachloride (SnCl 4 ·5H 2 O), and stirred to make it dissolve, L-cysteine and SnCl in the mixed solution 4 The molar ratio is 8:1.
[0028] 2) The obtained mixed solution was transferred to a polytetrafluoroethylene liner reactor, sealed, and the reactor was kept at 220° C. for 8 hours, and then naturally cooled to room temperature. The precipitate was separated by centrifugation, washed thoroughly with deionized water and absolute ethanol, and dried in vacuum to obtain the SnS 2 Nanosheet anode materials. X-ray diffraction (XRD) analysis and transmission electron microscope (TEM) observation results show that the obtained product is SnS 2 Nanosheets (see Figure 4 )
[0029] 3) by the 3rd of embodiment 1) the method for step is assembled into test cell, and by the 3rd of embodiment 1) the test method test SnS of step 2 Electrochemical lithium storage performance of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com