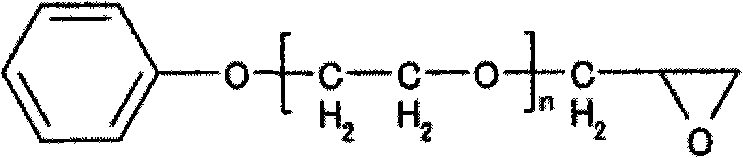
Process for producing photosensitive resin, photosensitive resin obtained by the process, and photosensitive resin composition
A technology of photosensitive resin and manufacturing method, which is applied in the field of photosensitive resin and photosensitive resin composition, can solve problems such as inability to develop with dilute alkali, poor solubility of dilute alkali, and drop in acid value, and achieve thermal stability and The effect of excellent development control latitude and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
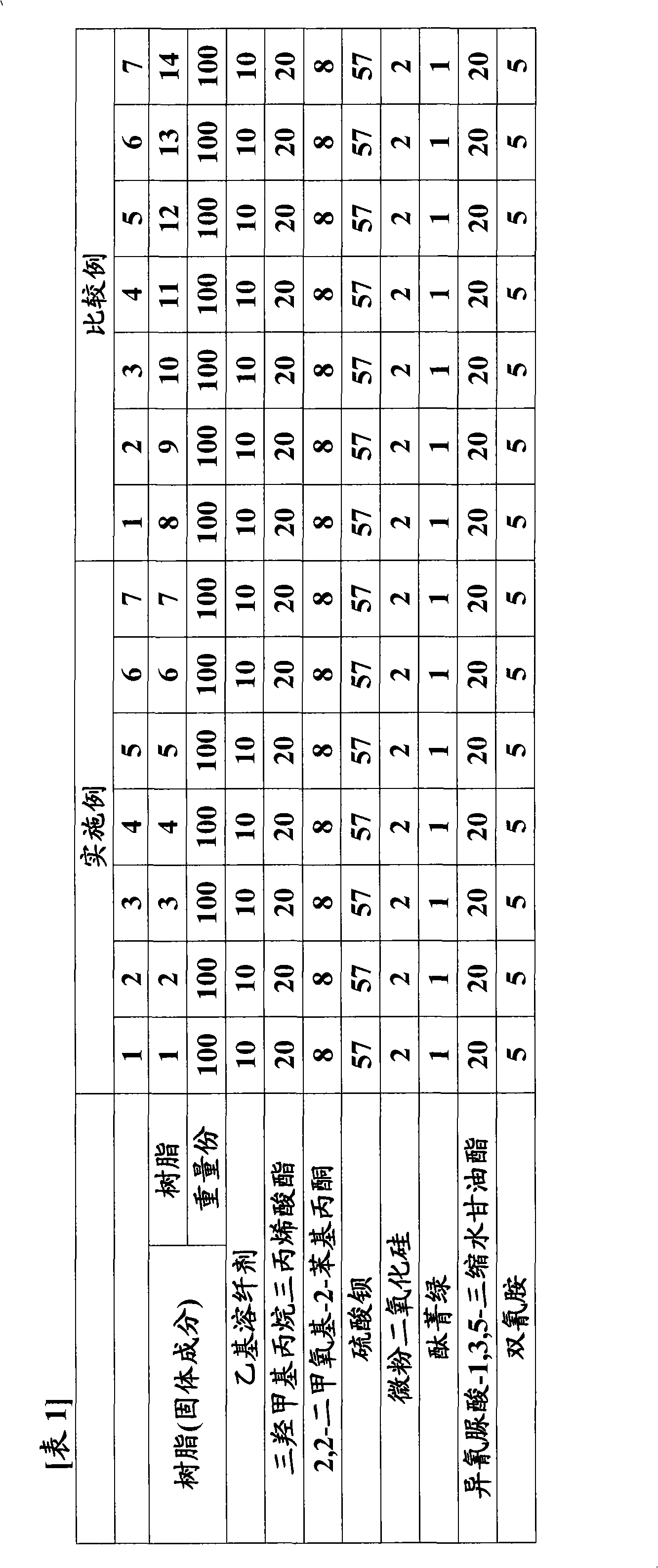
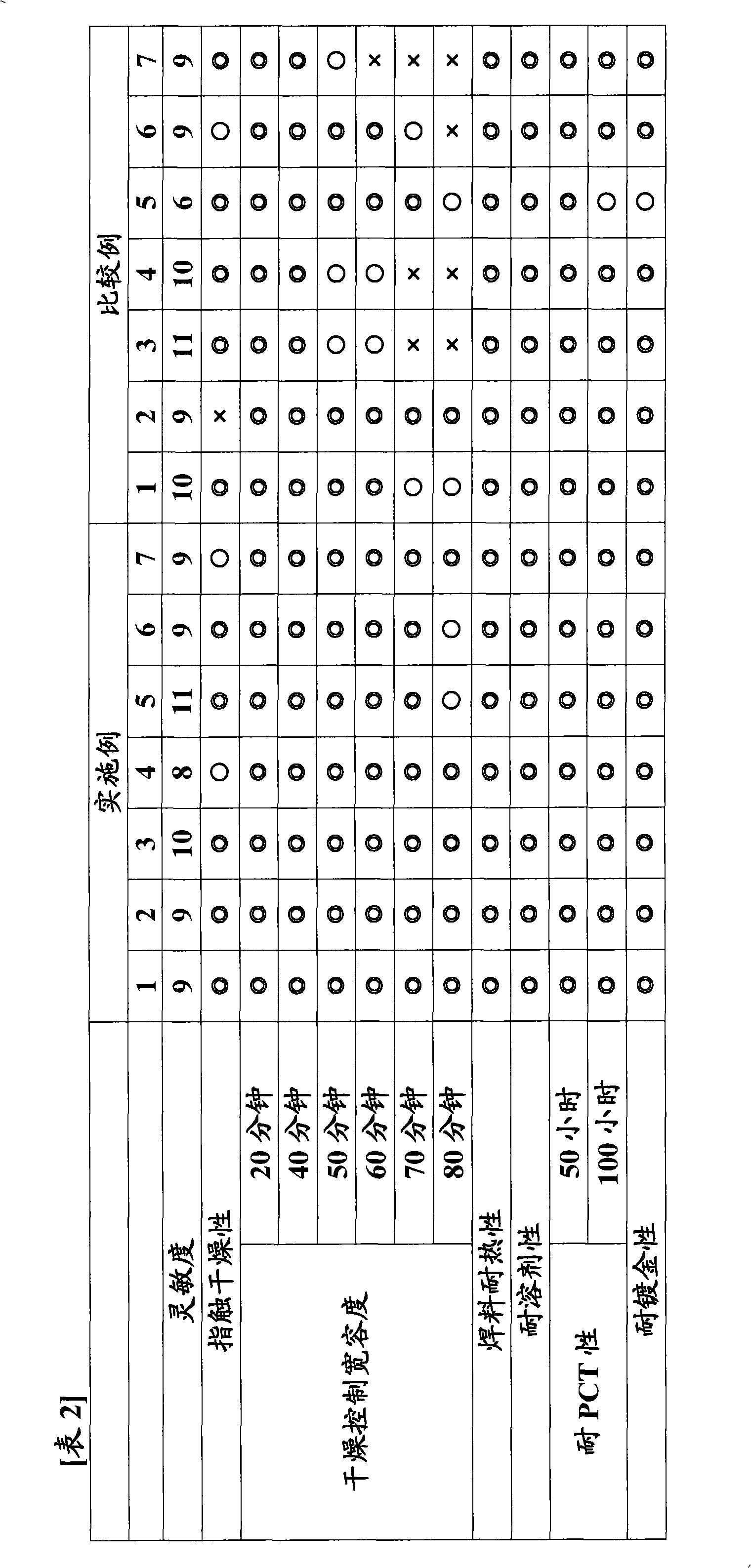
[0067] Hereinafter, the present invention will be described in more detail by way of Examples and Comparative Examples, and "parts" and "%" in each example are based on mass unless otherwise specified. The present invention is not limited to these examples.
Synthetic example 1
[0068] Synthesis Example 1 (Synthesis Example of Resin 1)
[0069] In the flask with stirrer, gas inlet tube, reflux tube, add 92 parts of ethyl carbitol acetate, dissolve 210 parts (1.0 equivalents) of o-cresol novolak type epoxy resin (trade name: エポトトト (registered trademark) YDCN704, manufactured by Tohto Chemical Co., Ltd., epoxy equivalent 210). Further add 69.84 parts (0.97 moles) of acrylic acid, 2.16 parts (0.03 moles) of glycolic acid, 2.17 parts of polymerization inhibitor 2,6-di-tert-butyl-4-methoxyphenol and 0.45 parts of triphenylphosphine, 0.85 parts of cyclo Chromium alkanoate (3% metal content). The reaction was continued for 10 hours while heating to 130° C. while blowing air from the gas inlet pipe at the lower part of the liquid surface to obtain a reactant (reactant I) having an acid value of 0.5 mg KOH / g. 98 parts of ethyl carbitol acetate and 106.4 parts (0.7 mol) of tetrahydrophthalic anhydride were added thereto, and the reaction was further carried ou...
Synthetic example 2
[0070] Synthesis Example 2 (Synthesis Example of Resin 2)
[0071] In the flask with stirrer, gas inlet tube, reflux tube, add 92 parts of ethyl carbitol acetate, dissolve 210 parts (1.0 equivalents) of o-cresol novolak type epoxy resin (trade name: エポトトト (registered trademark) YDCN704, manufactured by Tohto Chemical Co., Ltd., epoxy equivalent 210). Further add 69.84 parts (0.97 moles) of acrylic acid, 2.16 parts (0.03 moles) of glycolic acid, 2.17 parts of polymerization inhibitor 2,6-di-tert-butyl-4-methoxyphenol and 0.45 parts of triphenylphosphine, 0.85 parts of cyclo Zirconium alkanoate (metal content 6%) is heated to 130 ℃ and continued to react for 10 hours while blowing into air from the gas inlet pipe at the lower part of the liquid surface, to obtain the reactant (reactant I) that the acid value is 0.5mg KOH / g ). 98 parts of ethyl carbitol acetate and 106.4 parts (0.7 mol) of tetrahydrophthalic anhydride were added thereto, and the reaction was further carried out ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com