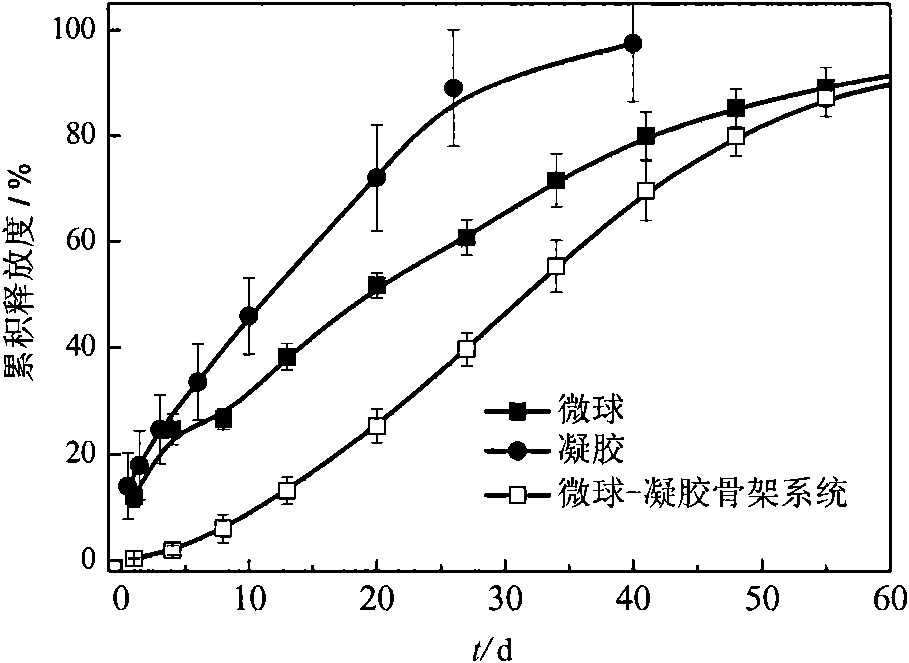
In-situ implantation drug delivery system of naltrexone microsphere-hydrogel matrix
A drug delivery system and technology of naltrexone, which is applied in the field of microsphere-hydrogel skeleton implanted drug delivery system in situ, can solve the problems of easy displacement and short duration, and reduce disintegration and release. The rate is stable and the effect of solving the problem of relapse
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
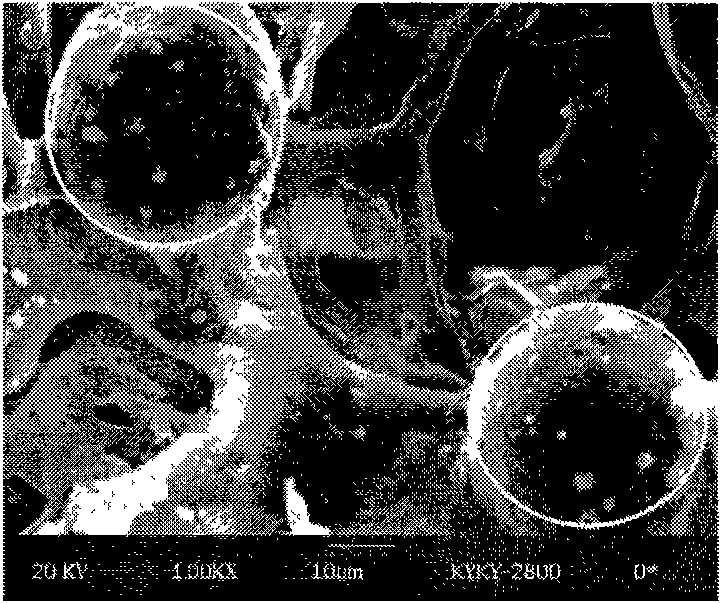
[0023] First, PLGA microspheres loaded with naltrexone were prepared. The substance quantity ratio of lactic acid and glycolic acid in the microsphere material PLGA is 50:50-75:25, and the molecular weight is 20k-80k. The mass ratio of the drug naltrexone and the carrier material lactic acid / glycolic acid copolymer in the microsphere is 1:1-1:6. The preparation method can adopt an O / W emulsification solvent evaporation method, the solvent can be dichloromethane, and the emulsification aid can be PVA. By adjusting the concentration of PLGA and PVA, microspheres with a particle size of 10-100 μm can be prepared. Microspheres can be stored lyophilized.
[0024] Then prepare methyl cellulose sol, its preparation method can refer to Chinese invention patent CN 1698902A. The sol is composed of methylcellulose (viscosity 400mPa·S), polyethylene glycol (molecular weight 11000), citrate and alginate (low viscosity), and the mass ratio of the four (wherein citrate and alginate As so...
Embodiment 1
[0028] This example shows the solidification characteristics of the naltrexone microsphere-hydrogel framework in situ implantation system.
[0029] The ratio of microspheres: lactic acid and glycolic acid in PLGA is 75:25, and the molecular weight is 80k. The mass ratio of the drug naltrexone and the carrier material lactic acid / glycolic acid copolymer in the microspheres is 1:2. The average particle size of the microspheres is 52±2 μm, and the encapsulation efficiency is 95%.
[0030] Gel skeleton: methylcellulose: polyethylene glycol: citrate: alginate = 2:8:1:3.5, the content of methylcellulose is 20mg methylcellulose per mL of hydrosol.
[0031] The content of microspheres in the gel matrix is: 30mg / mL
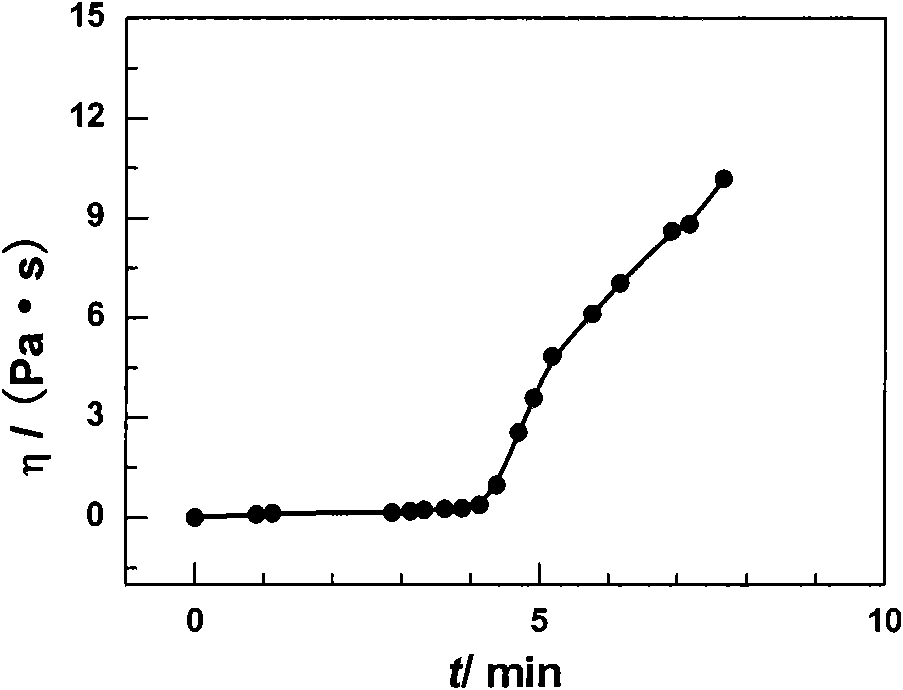
[0032] The viscosity change curve of the naltrexone microsphere-hydrogel matrix in situ implantation system at body temperature is as follows: figure 1 As shown, the curve is divided into two sections of slow speed and fast increase. The time corresponding to the inters...
Embodiment 2
[0034]This example is the effect of microsphere content in the naltrexone microsphere-hydrogel framework in situ implantation system on the solidification characteristics. The microspheres and gel skeleton are the same as in Example 3. The contents of microspheres in the gel matrix are respectively: 10mg / mL, 30mg / mL and 50mg / mL microsphere contents are different, and the solidification time of the naltrexone microsphere-hydrogel matrix in situ implantation system at body temperature is listed in the table 1. Although the coagulation time increased with the increase of the microsphere content, but when the microsphere content reached 50mg / mL, the coagulation time was still within 10min.
[0035] Table 1 The coagulation time of naltrexone microspheres-hydrogel matrix system at body temperature at different times
[0036] Microsphere content / mg·mL -1
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com