Fluorescent probe for selectively detecting zinc ions in cells, synthesizing method thereof and application thereof
A technology of fluorescent probes and zinc ions, applied in measuring devices, material analysis through optical means, instruments, etc., can solve the problems that chemical sensing molecules are rarely reported, and achieve simple structure, high efficiency, and simple synthesis method Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 5.4g of 7-N, N-dimethylcoumarin-3-methylketone was dissolved in 10ml of dimethyl sulfoxide, and a solution of 4g of 2-hydrazinopyridine dissolved in 100ml of ethanol was slowly added dropwise under reflux. After reflux and stirring for 3 hours, the reaction was filtered to remove dimethyl sulfoxide and ethanol solvent, and the orange blocky crystal (I-1) was obtained after vacuum drying.
[0040] EI-MS, m / e, 350.1[M+1] + .λ ab. max / nm=470nm, Φ=0.08.
[0041]
[0042] Structure of fluorescent probe (I-1)
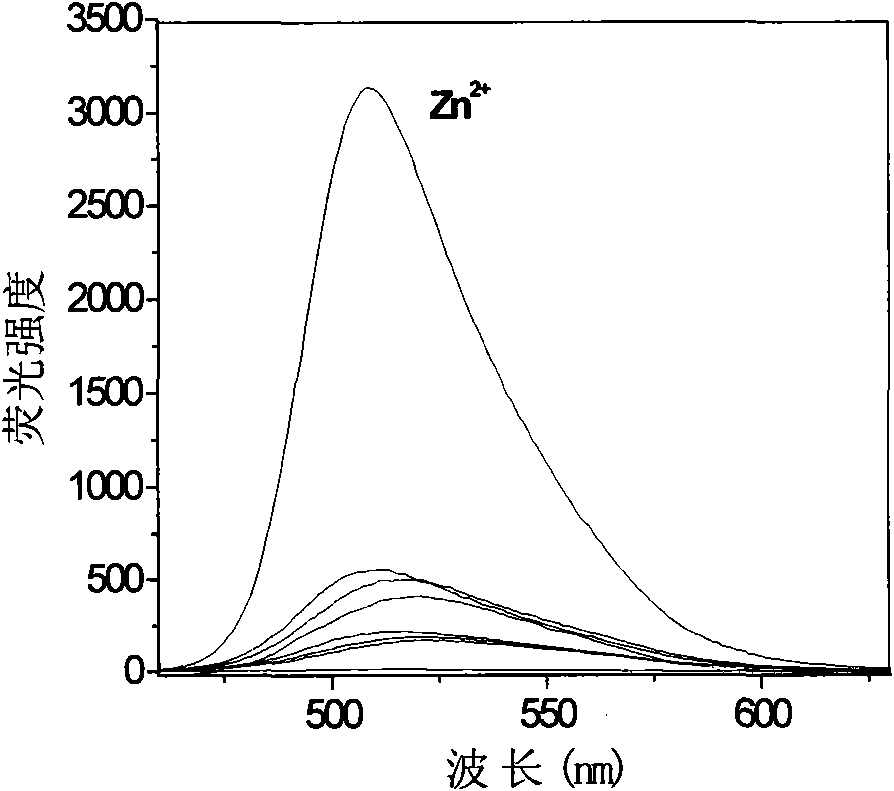
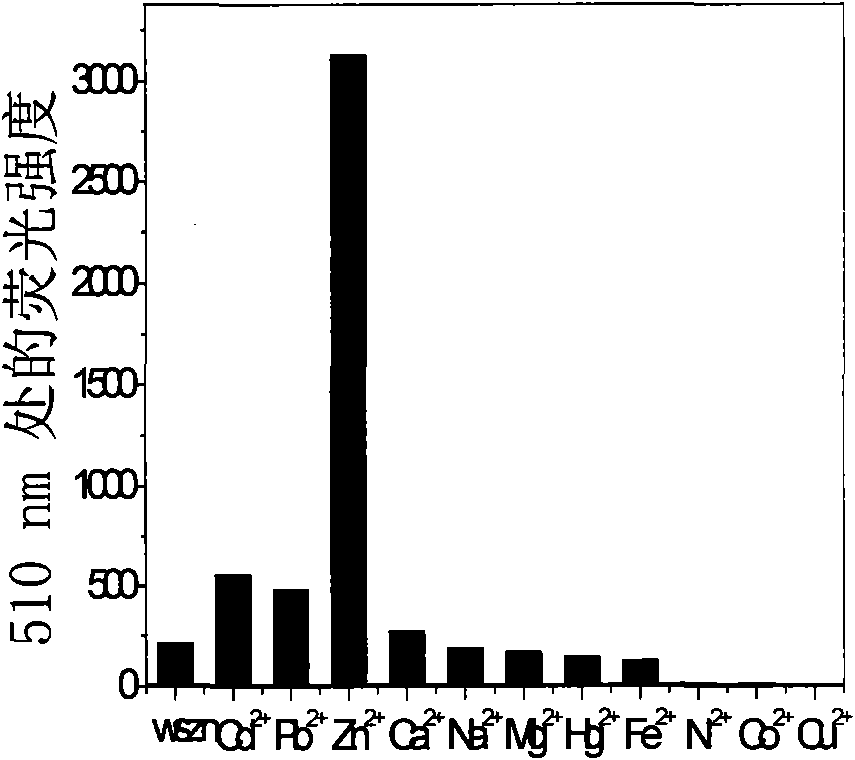
[0043] The fluorescent probe (I-1) was added to various metal ion aqueous solutions respectively, by Figure 1a , 1b can be seen that the fluorescent probe (I-1) on the zinc ion Zn 2+ There is a very specific fluorescent enhanced recognition effect.
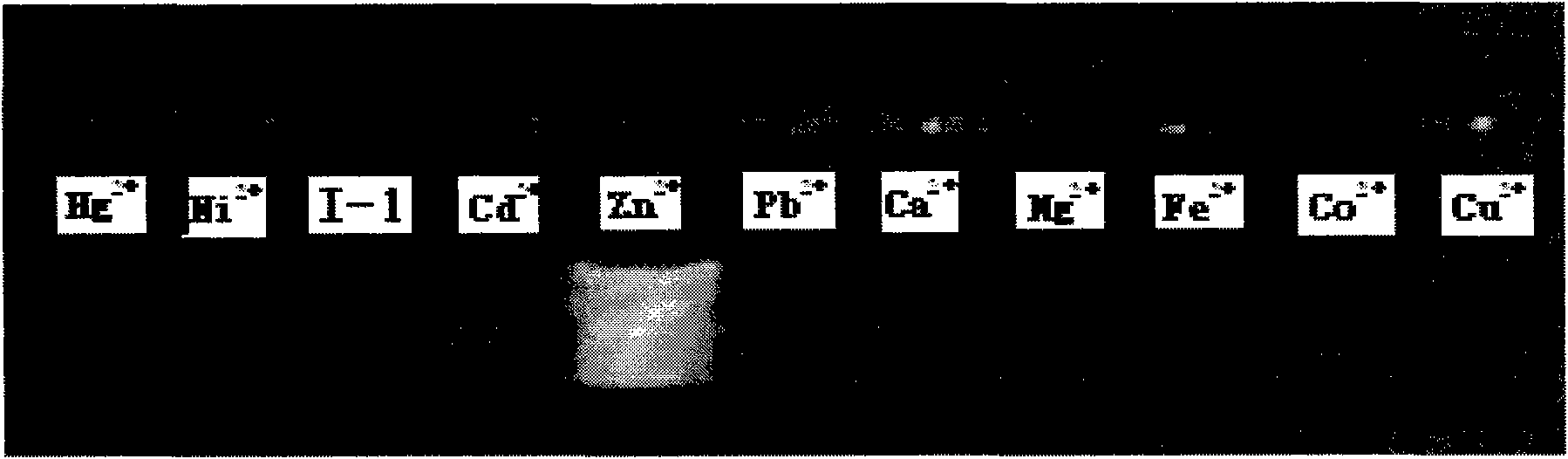
[0044] Fluorescent probes for zinc ions Zn 2+ For selective recognition see figure 2 From the photo, it can be seen that the fluorescence is greatly enhanced after adding zinc ions, but the change is not...
Embodiment 2
[0048] 5.6g 7-N, N-dimethylcoumarin-3-methyl ketone was dissolved in 10ml tetrahydrofuran, and slowly added dropwise a solution of 7g 2-hydrazino-4-methoxypyridine dissolved in 100ml acetonitrile under reflux . After stirring and reacting under reflux for 5 hours, the mixture was filtered to remove tetrahydrofuran and acetonitrile solvent, and dried in vacuo to obtain orange blocky crystals (I-2).
[0049] EI-MS, m / e, 380.1[M+1] + .λ ab. max / nm=470nm, Φ=0.08.
[0050]
[0051] Structure of fluorescent probe (I-2)
Embodiment 3
[0053] 5.4g of 7-N, N-dimethylcoumarin 4-ethyl-3-aldehyde was dissolved in 10ml of ethanol, and a solution of 4g of 2-hydrazinopyridine dissolved in 200ml of ethanol was slowly added dropwise under reflux. After reflux and stirring for 5 hours, the reaction was filtered, the ethanol solvent was removed, and the orange block crystal (I-3) was obtained after vacuum drying.
[0054] EI-MS, m / e, 363.1[M+1] + .λ ab. max / nm=478nm, Φ=0.12.
[0055]
[0056] Structure of fluorescent probe (I-3)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com