HCL ceftizoxime alapivoxil synthesizing process
A technique for the synthesis of ceftizoxime hydrochloride and a synthetic process, which is applied in the field of synthetic process of ceftizoxime propivoxil hydrochloride, can solve the problems of many by-products and high cost, and achieve the effects of reliable purity, mild reaction conditions, and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
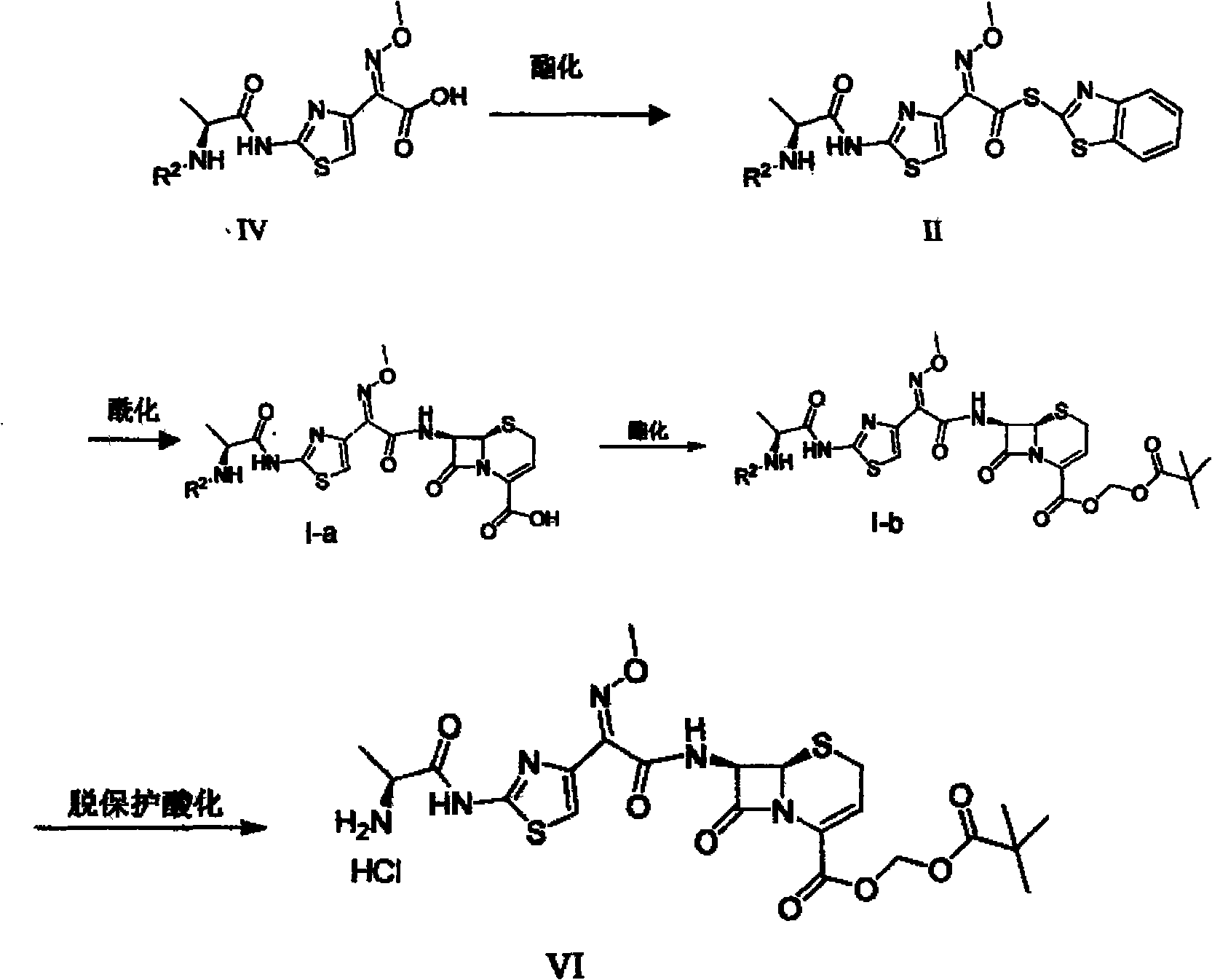
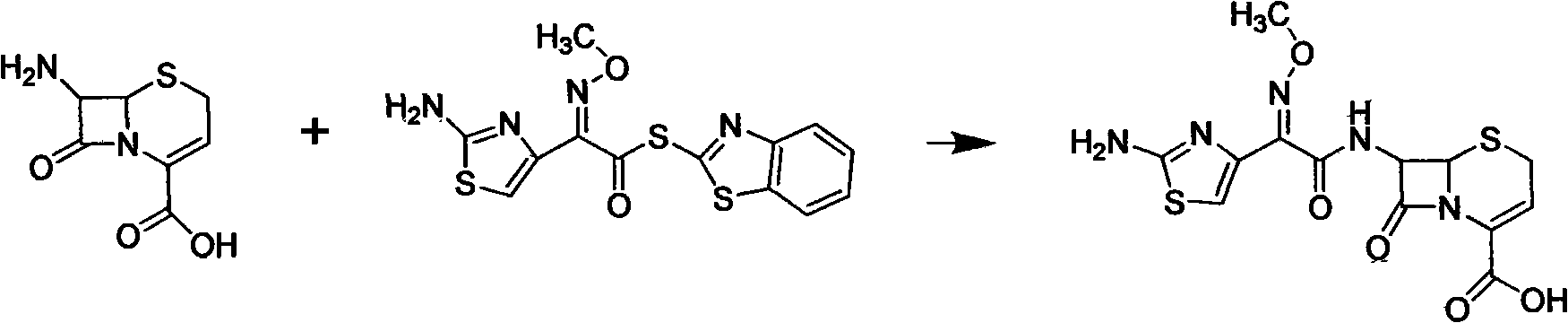
[0034] A kind of synthesis technique of cefazolin hydrochloride propiproxil, (1) first prepare solid cefazoxime: weigh 0.04mol 7-ANCA 8g and 0.04mol AE active ester 12.21g, add 7-ANCA to 40ml acetone solvent 25ml of water and 25ml of ethyl acetate were used for washing, and the aqueous layer was adjusted to pH 5-6 with 6N hydrochloric acid. Acidic, stirred, filtered, and dried at 60° C. to obtain 13 g of cefizoxime as a light yellow powdery solid; the yield was 162.5%.
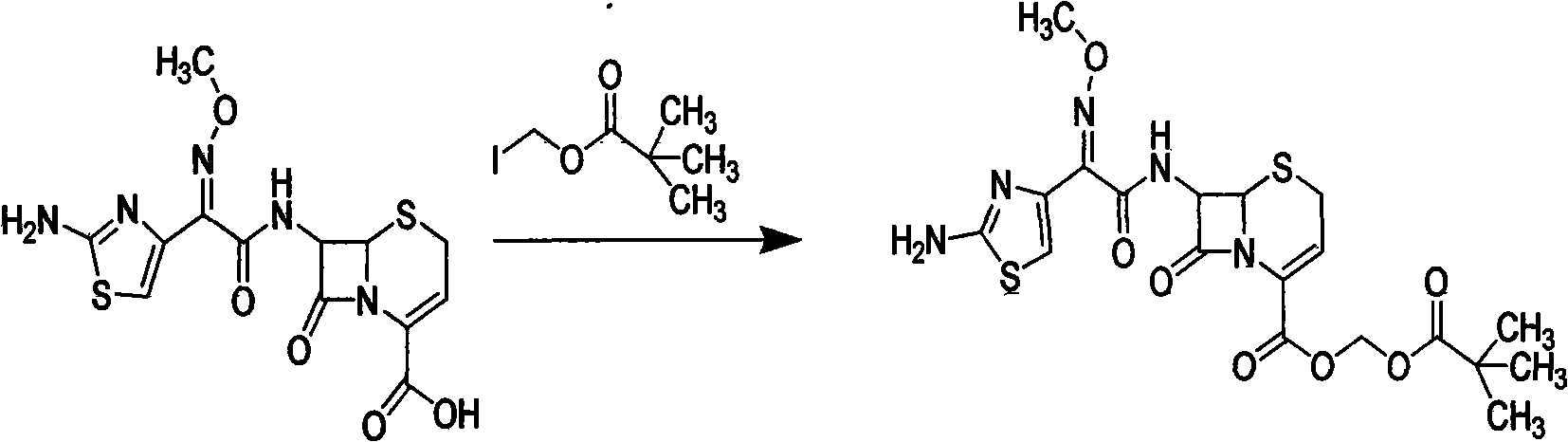
[0035] (2) Weigh 3.83 g (0.01 mol) of cefizoxime obtained in step (1), add 20 ml of DMF solvent, cool to 0° C., and carry out stirring reaction with 3 g of methyl iodide (0.012 mol) to become clear after 3 hours; Then 150 ml of ethyl acetate was added for extraction, and the organic layer was washed successively with sodium bicarbonate with a concentration of 1% by mass and sodium hydrogen sulfite solution with a concentration of 1% by mass, and each washing was repeated three times. Then, it was dried at 60°...
Embodiment 2
[0039] A kind of synthesis technique of cefazolin hydrochloride propiproxil, (1) first prepare solid cefazoxime: weigh 0.04mol 7-ANCA 8g and 0.04mol AE active ester 12.72g, add 7-ANCA to 40ml acetonitrile solvent , at room temperature, carry out stirring reaction with AE active ester for 5 hours, concentrate and remove acetone, and then carry out suction washing through 25ml water and 25ml ethyl acetate as the washing solution, and the aqueous layer is adjusted with 6N hydrochloric acid. The pH value is slightly acidic at 5-6. , stirred, filtered, and dried at 60° C. to obtain 11.81 g of cefizoxime as a light yellow powdery solid; the yield was 147.6%.
[0040] (2) Weigh 3.83 g (0.01 mol) of cefizoxime obtained in step (1), add 20 ml of tetrahydrofuran solvent, cool to 0°C, and carry out stirring reaction with methyl iodide 1.94 g (0.008 mol) for 3 hours, and then it becomes clear Then add 150ml ethyl acetate to extract, the organic layer is washed successively with the sodium...
Embodiment 3
[0044] A kind of synthesis technique of cefizoxime hydrochloride propiproxil, (1) first prepare solid cefizoxime: weigh 0.04mol 7-ANCA 8g and 0.1mol AE active ester 30.52g, add 7-ANCA to 40ml methanol solvent , at room temperature, carry out stirring reaction with AE active ester for 7.5 hours, concentrate and remove acetone, then carry out suction washing through 25ml water and 50ml ethyl acetate as washing liquid, and the aqueous layer is adjusted with 9N hydrochloric acid to pH value at 5-6 slightly acidic , stirred, filtered, and dried at 60° C. to obtain 12.64 g of ceftizoxime as a light yellow powdery solid; the yield was 157.9%.
[0045] (2) Weigh 3.83 g (0.01 mol) of cefizoxime obtained in step (1), add 20 ml of dimethyl sulfoxide solvent, cool to 0°C, and carry out stirring reaction with 3.39 g (0.014 mol) of methyl iodide for 4.5 hours Then it became clear; then 150 ml of ethyl acetate was added for extraction, and the organic layer was washed successively with 1% so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com