Aminosugar derivate, preparation method and medical application thereof
A drug and pharmaceutical technology, applied in the field of inhibition of neovascularization of chicken embryo allantoic membrane, compound N--3--acrylamide, can solve the complex structure of carbohydrate compounds, difficult industrial production, difficult analysis and preparation, etc. problem, to achieve a strong effect of chick embryo allantoic membrane neovascularization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
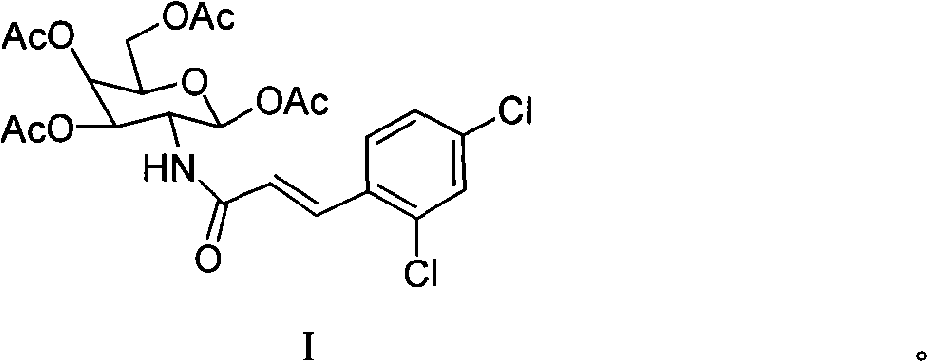
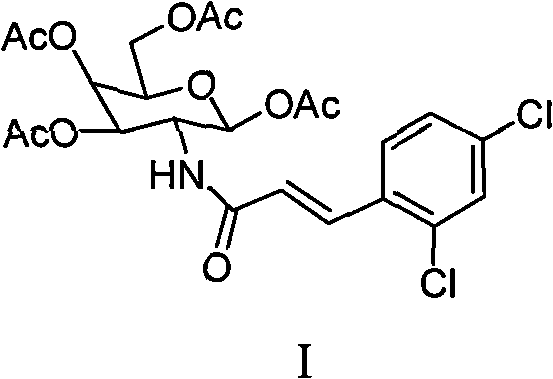
[0040] N-(2,3,4,6-tetra-O-acetyl-1-deoxy-β-D-galactopyranosyl)-3-(2,4-dichlorophenyl)-2-acrylamide Preparation of (I) 2-benzylidene-1,3,4,6-tetrahydroxy-2-deoxy-β-D-galactopyranose (4)
[0041] Add 10g (46mmol) of 1,3,4,6-tetrahydroxy-2-deoxy-D-galactopyranosamine hydrochloride to 47mL aqueous solution containing 2.2g (55mmol) of NaOH at 0°C, under mechanical stirring Slowly add 5.4mL (53mmol) of benzaldehyde, a white solid precipitates out quickly, continue to stir for 1h and then stop the reaction, place it at 0°C for 12h, filter, then mix with water, V (ether): V (ethanol) = 4:1 Washed with solvent and dried under infrared light to obtain 10 g of white solid mixture, which was directly used in the next reaction without purification.
[0042] 2-Benzylidene-1,3,4,6-tetra-O-acetyl-2-deoxy-β-D-galactopyranose (5)
[0043] Add 10 g of the mixture containing intermediate 4 into 60 mL of pyridine, add 42 mL (0.46 mol) of acetic anhydride while stirring in an ice-water bath, rais...
Embodiment 2
[0056] tablet
[0057] Get compound I 500mg obtained in Example 1, starch 2g, dextrin 1g mix, make soft material with appropriate amount of 30% ethanol as wetting agent, conventional method granulate, add appropriate amount of magnesium stearate, make tablet.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com