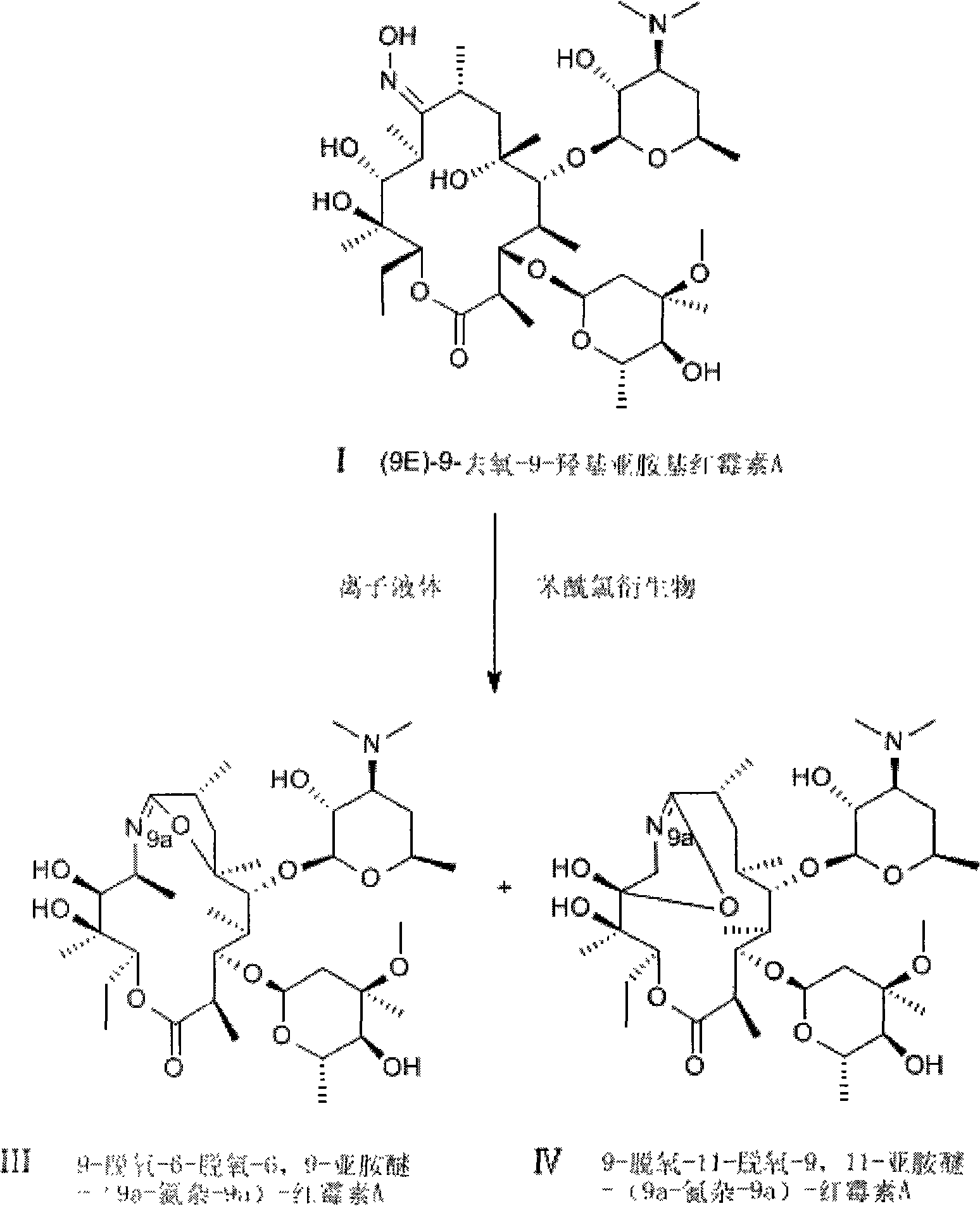
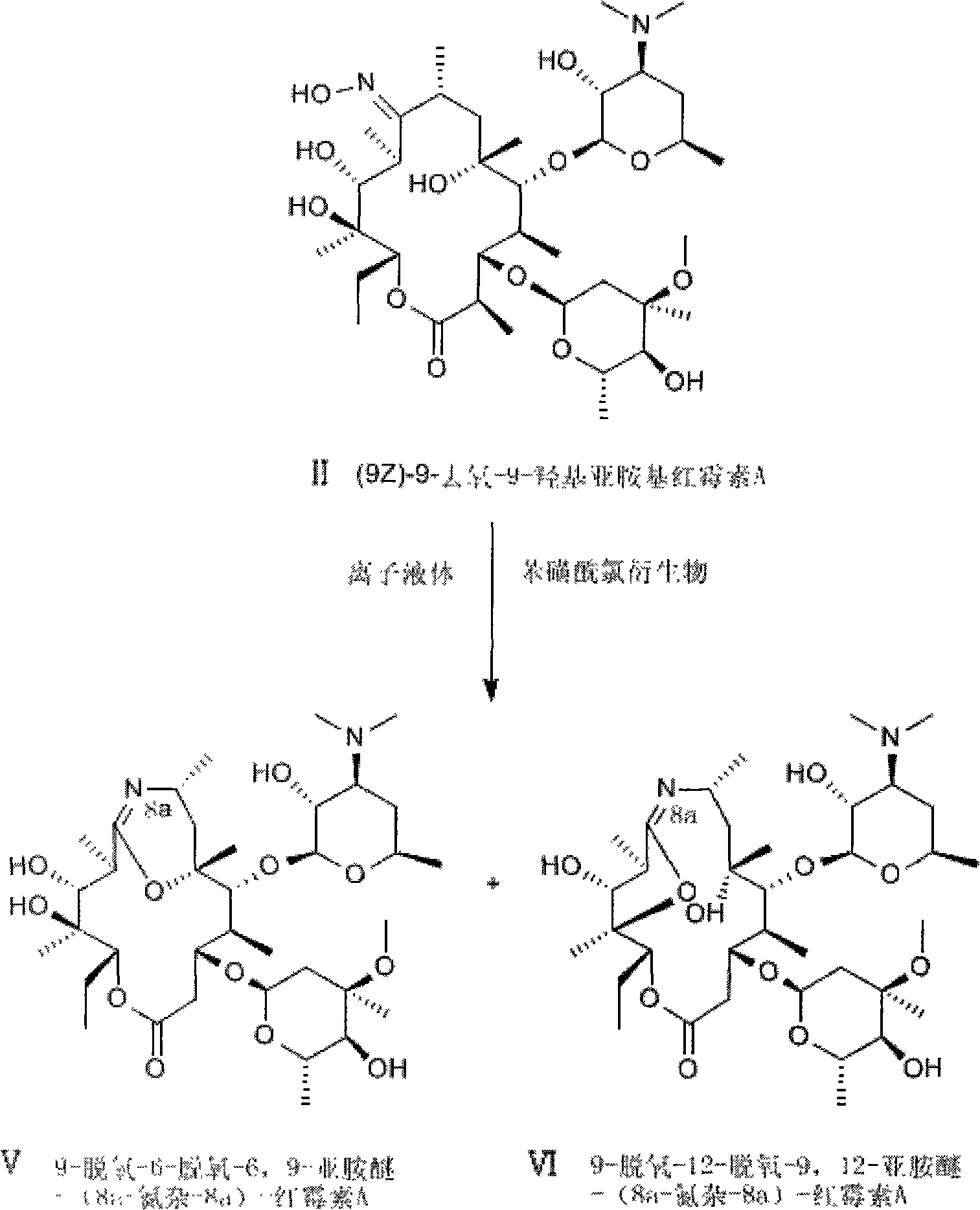
Method for preparing erythrocin A iminoether salt
The technology of erythromycin and imine ether is applied in the field of preparation of erythromycin A imine ether salt, which can solve the problems of low bioavailability and difficult control of dosage, and achieve the effect of saving resources, simple device and reaction system.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add p-toluenesulfonyl chloride 0.038g (0.2mmol) in a 25ml round bottom flask, add 0.15g (0.2mmol) (9E)-9-deoxy-9-hydroxyimino erythromycin A, p-toluenesulfonyl chloride and (9E)-9-deoxy-9-hydroxyiminoerythromycin A molar ratio is 1:1, then add 11.5g ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate (40mmol), The molar ratio of room temperature ionic liquid to (9E)-9-deoxy-9-hydroxyiminoerythromycin A is 200:1, put in a magnetic stirrer, stir at 5°C until dissolved, and stop the reaction after 3 hours of reaction . Add 20ml of water three times to extract the ionic liquid phase, layered, add 30ml of dichloromethane to extract the aqueous phase, layered, add an appropriate amount of anhydrous magnesium sulfate to the organic phase to dry, filter, and evaporate the organic phase to dryness with a rotary evaporator. 0.138 g of white powder was obtained with a conversion rate of 92%. The melting point was measured to be 115°C-120°C. HPLC quantitatively measures...
Embodiment 2
[0028] Add p-toluenesulfonyl chloride 0.076g (0.4mmol) in a 25ml round bottom flask, add 0.15g (0.2mmol) (9E)-9-deoxy-9-hydroxyimino erythromycin A, p-toluenesulfonyl chloride and (9E)-9-deoxy-9-hydroxyiminoerythromycin A molar ratio is 2:1, then add 11g ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate (40mmol), ion The molar ratio of the liquid to (9E)-9-deoxy-9-hydroxyiminoerythromycin A is 200:1, put in a magnetic stirrer, stir at 0°C until dissolved, and stop the reaction after 1.5 hours of reaction. Add 20ml of water three times to extract the ionic liquid phase, layered, add 30ml of dichloromethane to extract the aqueous phase, layered, add an appropriate amount of anhydrous magnesium sulfate to the organic phase to dry, filter, and evaporate the organic phase to dryness with a rotary evaporator. Obtained 0.141g of white powder, the conversion rate was 94%, and the recorded melting point was 117°C-121°C. HPLC quantitatively measured 9-deoxy-6-deoxy-6,9-imino ...
Embodiment 3
[0030]Add 0.066g (0.3mmol) of p-nitrobenzenesulfonyl chloride to a 25ml round bottom flask, add 0.075g (0.1mmol) of (9E)-9-deoxy-9-hydroxyiminoerythromycin A, p-nitro The molar ratio of benzenesulfonyl chloride to (9E)-9-deoxy-9-hydroxyiminoerythromycin A is 3:1, then add 5.1g of ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate (18mmol), the molar ratio of ionic liquid to (9E)-9-deoxy-9-hydroxyiminoerythromycin A is 180:1, put in a magnetic stirrer, stir at 5°C until dissolved, and react for 2.5 hours After stopping the reaction. Add 20ml of water three times to extract the ionic liquid phase, layered, add 30ml of dichloromethane to extract the aqueous phase, layered, add an appropriate amount of anhydrous magnesium sulfate to the organic phase to dry, filter, and evaporate the organic phase to dryness with a rotary evaporator. Obtained 0.069g of white powder, the conversion rate was 92%, and the recorded melting point was 115°C-120°C. HPLC quantitatively measured...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com