Method and device for preventing oxidation in synthesis of anode material of lithium-ion battery
A technology for lithium-ion batteries and cathode materials, which is applied in the field of anti-oxidation of cathode materials for synthesizing lithium-ion batteries, can solve the problems of large amount of protective gas, unstable product performance, and unfavorable large-scale production, so as to reduce the purity of equipment and protective atmosphere. requirements, the requirements for reducing air tightness, and the effect of reasonable structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
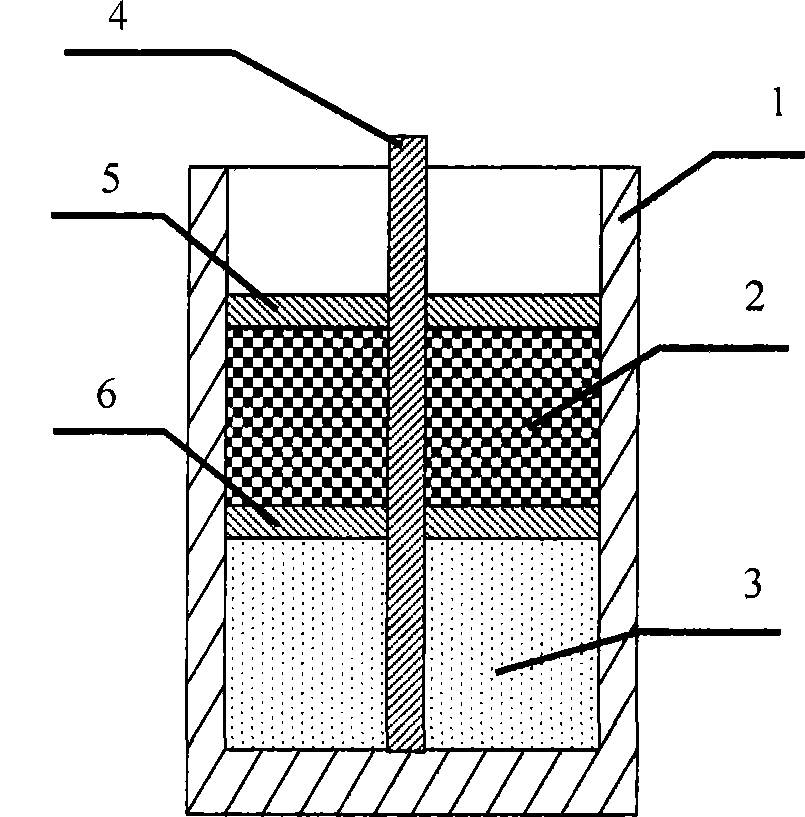
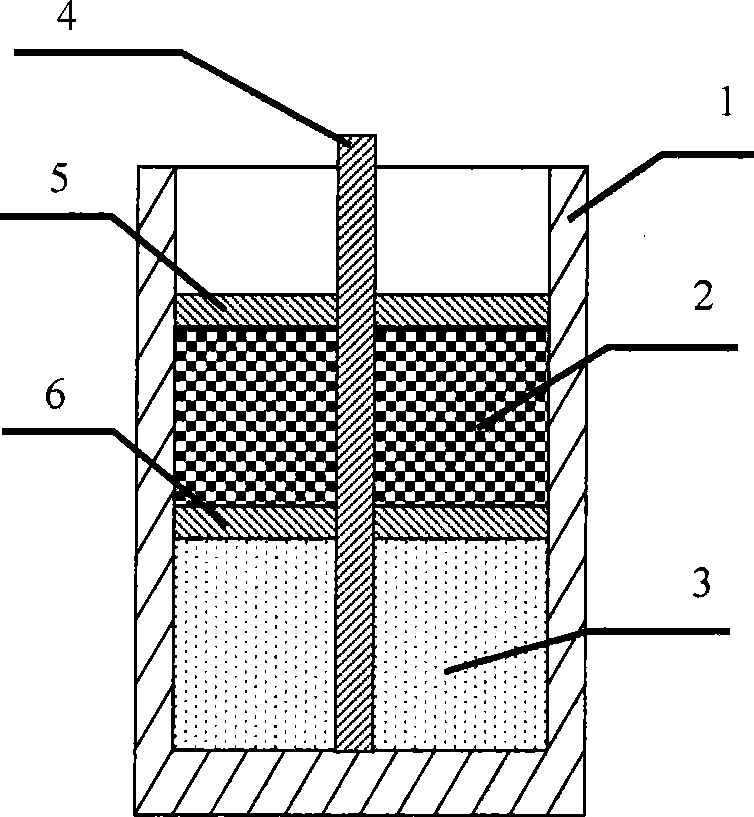
[0042] The schematic diagram of the reaction vessel is shown in figure 1 , figure 2 shown. The reactant ammonium dihydrogen phosphate, ferrous oxalate, lithium carbonate, and conductive agent required for the synthesis of lithium iron phosphate, the positive electrode material of the lithium ion battery, are filled in the loading area of the reaction vessel. Place a partition on the upper surface of the loading area. Graphite, a reductant used to prevent oxidation of reactants and products in the loading zone, is placed in the isolation zone. Place a cover plate on the upper surface of the isolation area. Inside the reaction vessel, an upright fixing rod is welded at the bottom to fix the cover and partition. The filled reaction container is put into an electric furnace for high-temperature sintering reaction, and the atmosphere in the reaction zone inside the electric furnace is a mixed gas of oxygen and nitrogen with an oxygen content of 0.5%, 4%, or 20%. Raise the t...
Embodiment 2
[0045] The schematic diagram of the reaction vessel is shown in image 3 , Figure 4 shown. The reactant ammonium dihydrogen phosphate, manganese carbonate, lithium carbonate and conductive agent required for synthesizing lithium manganese phosphate lithium ion battery cathode material are filled in the loading area of the reaction vessel. Place a partition on the upper surface of the loading area. A mixture of reducing agent acetylene black, activated carbon and phenolic resin is placed in the isolation area to prevent the reactants and products in the loading area from being oxidized. Place a cover plate on the upper surface of the isolation area. Inside the reaction vessel, an upright fixing rod is welded at the bottom to fix the cover and partition. The filled reaction container is put into an electric furnace for high-temperature sintering reaction, and the atmosphere in the reaction zone inside the electric furnace is a mixed gas of oxygen and argon with an oxygen ...
Embodiment 3
[0048] The schematic diagram of the reaction vessel is shown in Figure 5 , Figure 6 shown. The reactant lithium carbonate, ammonium dihydrogen phosphate, vanadium phosphate and conductive agent required for the synthesis of lithium vanadium phosphate, the positive electrode material of the lithium ion battery, are filled in the loading area of the reaction vessel. Place a partition on the upper surface of the loading area. A reducing agent metal aluminum for preventing oxidation of reactants and products in the loading area is placed in the isolation area. Place a cover plate on the upper surface of the isolation area. Inside the reaction vessel, an upright fixing rod is welded at the bottom to fix the cover and partition. Put the filled reaction container into the electric furnace for high-temperature sintering reaction, and the atmosphere in the reaction zone inside the electric furnace is air. Raise the temperature of the electric furnace to 650° C., keep the tempe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com