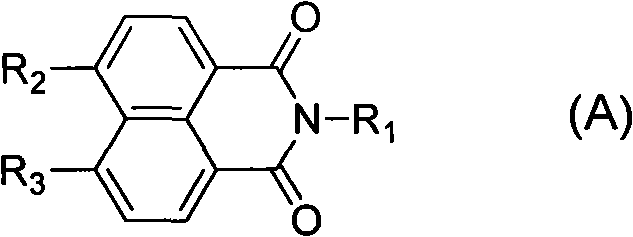
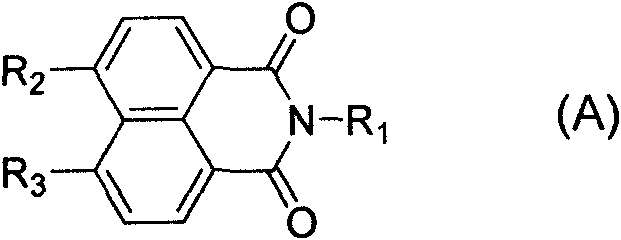
Anti-tumor compound containing triazole heterocyclic structure and application thereof
A triazole and compound technology, applied in the field of medicinal chemistry, can solve the problems of limited clinical activity, bone loss, unsatisfactory performance of amonafide, etc., and achieve strong anti-tumor activity and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
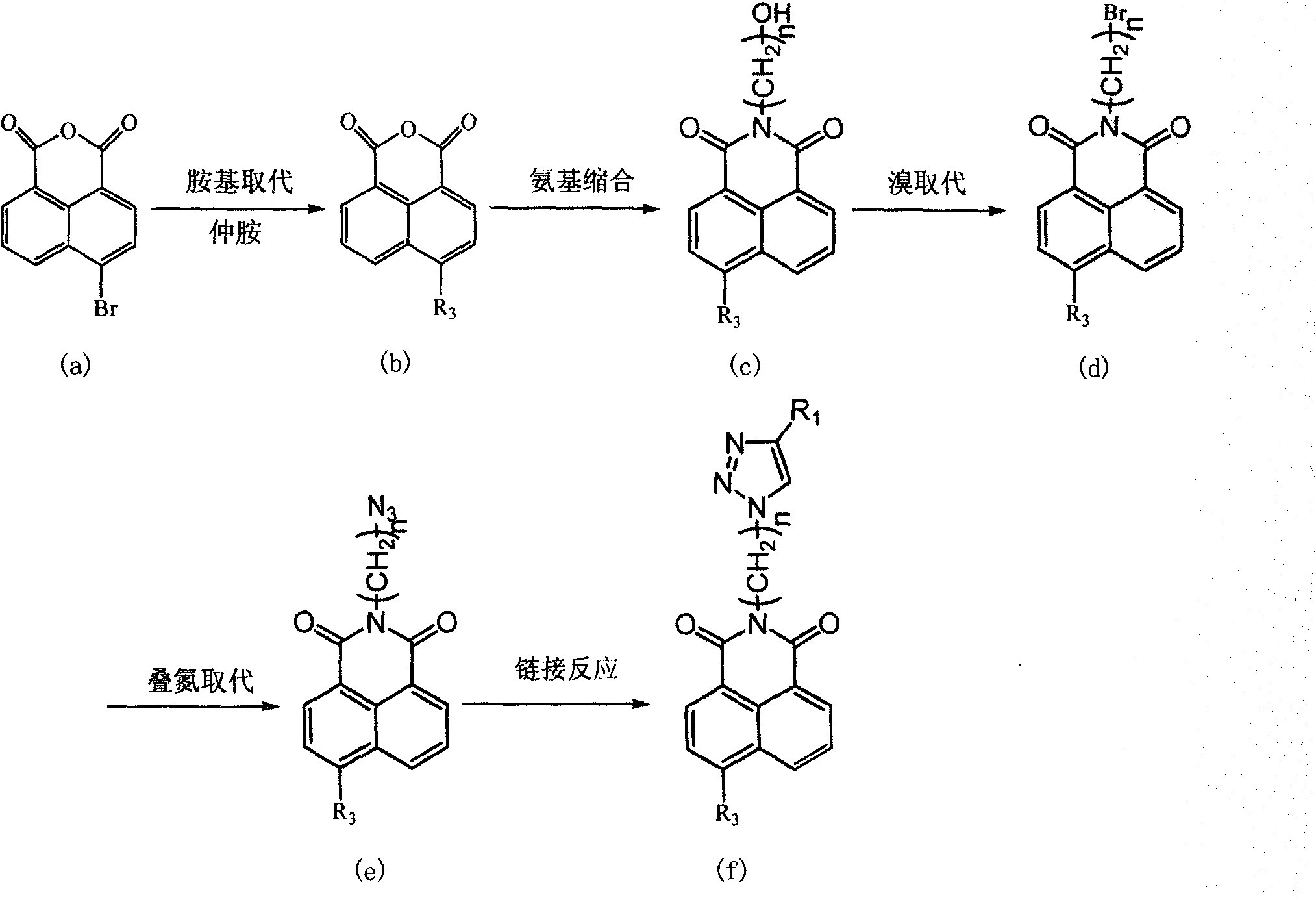
[0024] Synthesis of N-[3'-(4-phenyl-[1,2,3]-triazole)-propyl]-4-morpholinyl-1,8-naphthalimide (compound 1):
[0025] (1) 2.77 g of 4-bromo-1,8 naphthalene anhydride (0.01 mol) was added to 20 ml of ethylene glycol monomethyl ether, and 2.6 ml of morpholine was added thereto. The temperature was raised, refluxed, and the reaction was followed by thin plate chromatography until the reaction was complete, left standing overnight, filtered, and the solid was weighed to be 2.16 g, and the yield was 76.5%.
[0026]
[0027] (2) Take 2 g of the product synthesized in step (1), add it to 30 ml of absolute ethanol, and then add 0.69 g of n-propanolamine (0.7 ml). The temperature was raised, refluxed, and the reaction was followed by thin-plate chromatography until the reaction was complete, left standing, and filtered to obtain 2.0 g of a yellow solid.
[0028]
[0029] (3) get 1.5 grams of the product synthesized by step (2), add in 15ml of ethyl acetate, slowly drip 0.8ml of P...
Embodiment 2
[0038] Synthesis of N-[3'-(4-phenyl-[1,2,3]-triazole)-propyl]-4-thiomorpholine-1,8-naphthalimide (Compound 2) :
[0039] Except for replacing the morpholine ring with thiomorpholine, other synthesis and purification methods are the same as in Example 1, to obtain the target compound 2
[0040] 1HNMR(d6-CDCl 3 , 400MHz): δ (ppm): 2.46 (m, 2H), 2.97 (t, J 1 =4Hz, J 2 =4.4Hz, 4H), 3.50(t, J 1 =4.4Hz, J 2 =4.0Hz, 4H), 4.31(t, J 1 =6.4Hz, J 2 =6.8Hz, 2H), 4.54(t, J 1 =6.8Hz, J 2 =7.2Hz, 2H), 7.22(d, J=8.0Hz, 1H), 7.32(t, J 1 =6.8Hz, J 2 =7.6Hz, 1H), 7.42(t, J 1 =7.6Hz, J 2 =7.6Hz, 2H), 7.71(t, J 1 =8.0Hz, J 2 =7.6Hz, 1H), 7.80(d, J=8.0Hz, 2H), 8.04(s, 1H), 8.36(d, J=8.4Hz, 1H), 8.51(d, J=8.0Hz, 1H), 8.59 (d, J=7.2 Hz, 1H).
[0041] HR-MS (m / z): C 27 H 25 N 5 O 2 S, calculated: 483.1729, found: 483.1728.
Embodiment 3
[0043] Synthesis of N-[3'-(4-phenyl-[1,2,3]-triazole)-propyl]-4-hexahydropyridine-1,8-naphthalimide (compound 3):
[0044] Except for replacing the morpholine ring with hexahydropyridine, other synthesis and purification methods are the same as those in Example 1, to obtain the target compound 3
[0045] 1HNMR(d6-CDCl 3 , 400MHz): δ (ppm): 1.89 (m, 4H), 2.45 (m, 2H), 3.24 (t, J 1 =4.0Hz, J 2 =4.4Hz, 4H), 3.73(m, 2H), 4.31(t, J 1 =6.4Hz, J 2 =6.4Hz, 2H), 4.54(t, J 1 =7.2Hz, J 2 =6.8Hz, 2H), 7.17(d, J=8.4Hz, 1H), 7.32(t, J 1 =7.2Hz, J 2 =7.2Hz, 1H), 7.42(t, J 1 =7.6Hz, J 2 =7.2Hz, 2H), 7.68(t, J 1 =8.0Hz, J 2 =7.6Hz, 1H), 7.80(d, J=8.0Hz, 2H), 8.04(s, 1H), 8.39(d, J=8.4Hz, 1H), 8.49(d, J=8.4Hz, 1H), 8.58 (d, J=7.2 Hz, 1H).
[0046] HR-MS (m / z): C 28 H 27 N 5 O 2 , Calculated: 465.2165, Measured: 465.2162.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com