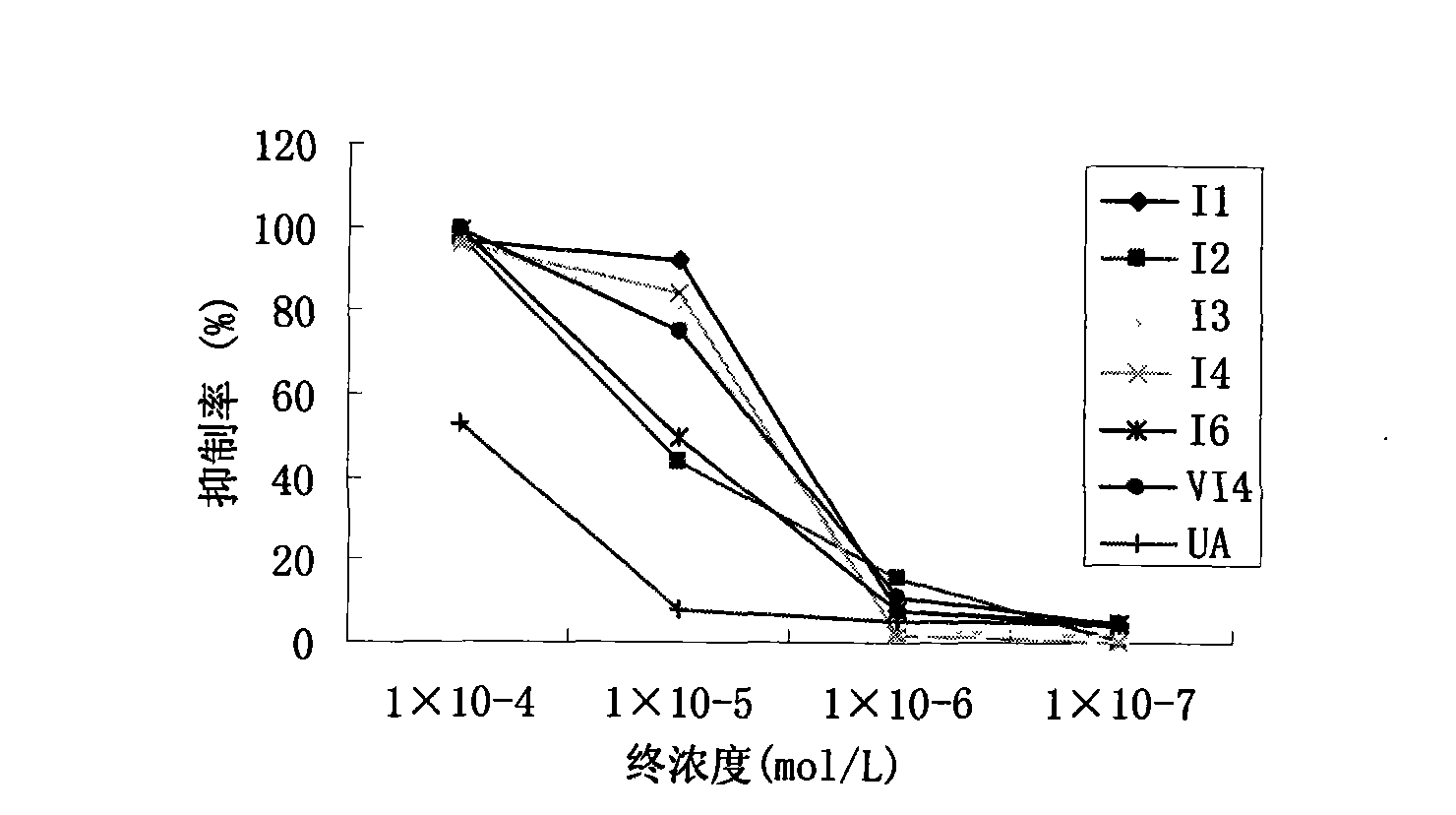
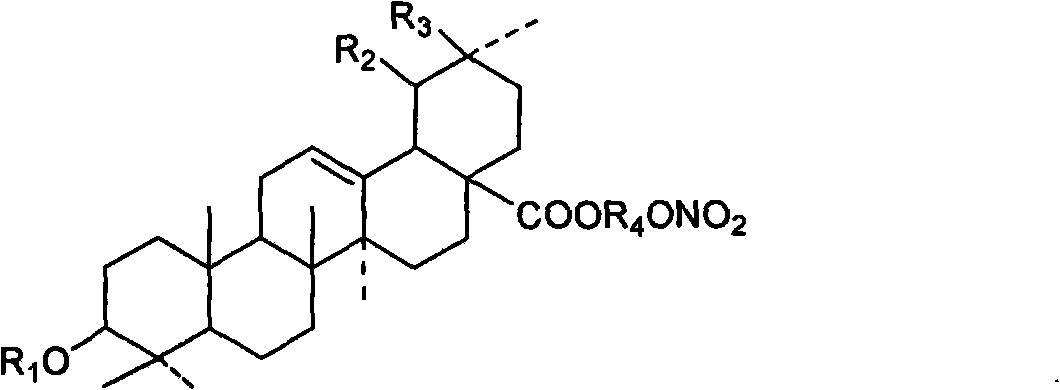
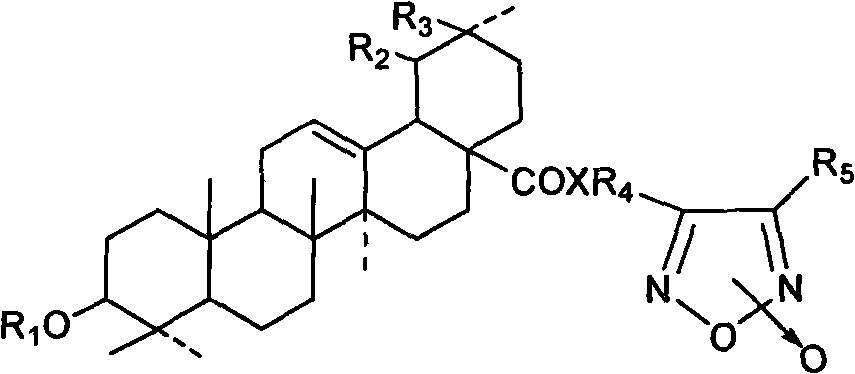
Novel tetraterpene derivatives and pharmaceutical use thereof
A compound, the technology of trifluoroacetyl group, applied in the application field of anti-liver tumor, can solve the problems of unsatisfactory curative effect, low bioavailability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] 1) ursolic acid-2-bromoethyl ester (1a 1 )
[0043] Suspend UA (1.0g, 2.19mmol) in 15ml of acetone, add triethylamine (2ml) to dissolve completely, add 1,2-dibromoethane (1.0ml, 11.5mmol) under stirring at room temperature, stir and reflux for 6 hours , filtered off the insoluble matter, concentrated the filtrate, and column chromatography [ethyl acetate:petroleum ether (60~90°C)=1:4(V:V)] gave a white solid 0.80g, yield 65%, mp: 150 ~152°C.
[0044] 2) ursolic acid-2-nitroxyethyl ester (I 1 )
[0045] 1a 1 (0.4g, 0.71mmol) was dissolved in a mixed solvent of acetonitrile and tetrahydrofuran, silver nitrate (177mg, 1.06mmol) was added, and the light was refluxed for 6 hours, the yellow silver bromide precipitate was filtered off, and the filtrate was concentrated to obtain a light yellow oil The product was subjected to column chromatography [ethyl acetate:petroleum ether (60-90°C)=1:4 (V:V)] to obtain 0.3g of pure product, yield 77.6%, mp: 178-180°C.
[0046] ESI...
Embodiment 2
[0052] Ursolic acid-3-(3-benzenesulfonyl-2-oxo-furazan-4-oxo)-1-methylpropyl ester (II 1 )
[0053] UA (456mg, 1mmol) was suspended in toluene, trifluoroacetic anhydride (0.82ml, 5.87mmol) was added, stirred at room temperature, and intermediate 2-(3-benzenesulfonyl-1,2,5-oxadiol) was added after 10 minutes Azole-2-oxide-4-)oxy-3-butanol (305mg, 1mmol) (refer to literature Li Ruiwen, Zhang Yihua, Ji Hui, etc. benzenesulfonyl furazan nitrogen oxide and diclofenac coupling compound Synthesis and anti-inflammatory activity [J]. Acta Pharmaceutica Sinica, 2001, 36 (11): 821-826), stirred and refluxed reaction, finished reaction after 6 hours, cooled, and the reaction solution was washed to neutrality with 10% NaOH, and the organic layer was Washed with water, saturated brine, anhydrous Na 2 SO 4 Drying, column chromatography [ethyl acetate: petroleum ether (60 ~ 90 ° C) = 1: 4 (V: V)], to obtain 523 mg of milky white solid, which was suspended in methanol, and sodium bicarbonat...
Embodiment 3
[0060] 1) 3-O-carboxypropionyl-ursolic acid (3a 1 )
[0061] UA (2.50g, 5.48mmol), succinic anhydride (2.19g, 21.9mmol), DMAP (0.668g, 5.48mmol), anhydrous CH 2 Cl 2 (60ml) was added in the reaction flask successively, and heated to reflux for 48 hours. The reaction solution was washed 3 times with water, and the organic layer was anhydrous Na 2 SO 4 Drying; filtration, concentration of the filtrate, and column chromatography of the concentrate [ethyl acetate:petroleum ether (60-90° C.)=1:4 (V:V)] yielded 1.696 g of the product, with a yield of 53%.
[0062] 2) 3-O-{4-[2-(2-nitrooxy-ethoxycarbonyl)-vinyl]-phenoxy}-succinyl-ursolic acid (III 1 )
[0063] Will 3a 1 (556mg, 1mmol), 2-bromoethyl 4-hydroxyphenylacrylate (260mg, 1mmol), DCC (206mg, 1mmol), and a catalytic amount of DMAP were added to the reaction flask, anhydrous CH 2 Cl 2 10ml, stirred at room temperature, filtered, the filtrate was concentrated under reduced pressure, and column chromatography [ethyl acet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com