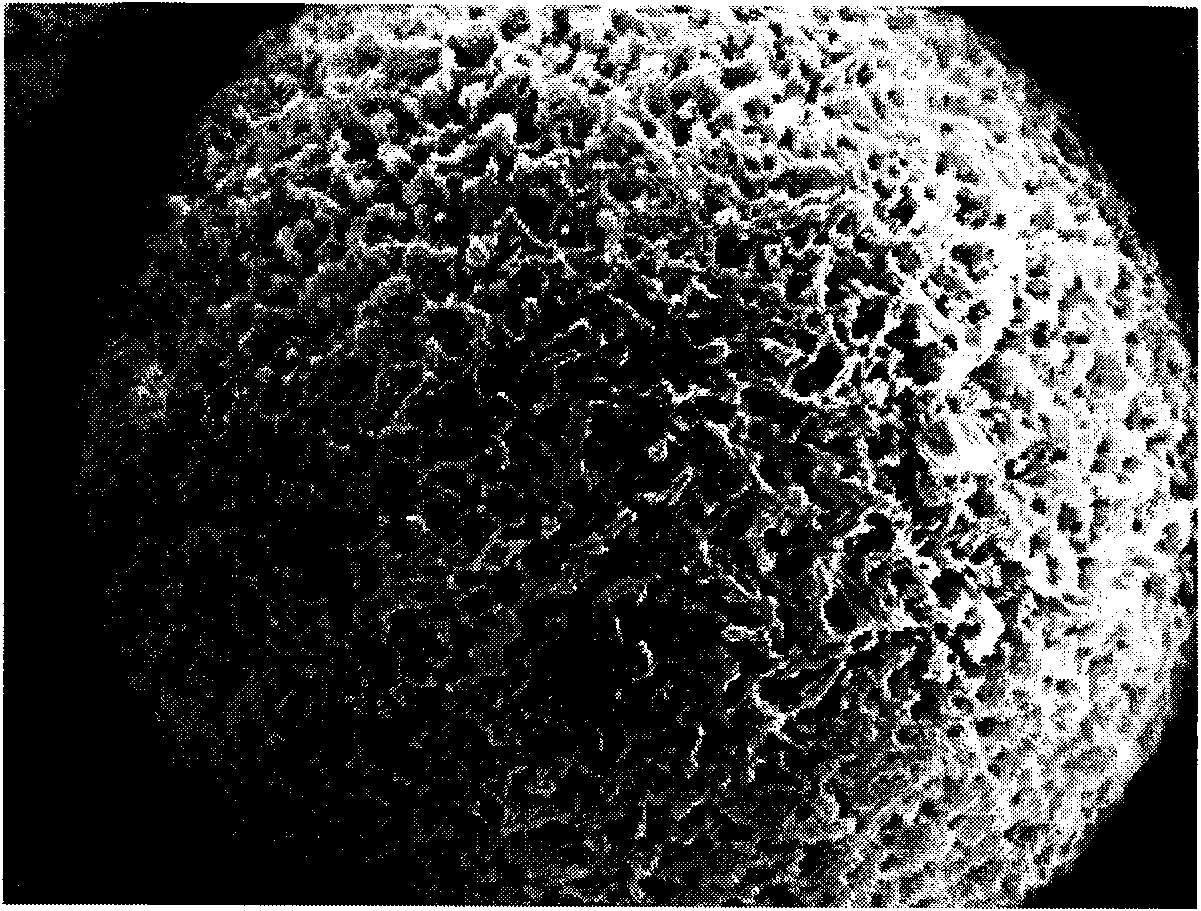Ultrasonically controlled-release target medicinal preparation and production method thereof
A technology of ultrasonic control and pharmaceutical preparations, which is applied in the directions of pharmaceutical formulations, medical preparations with non-active ingredients, non-active ingredients of polymer compounds, etc. Dosing and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1, preparation and performance of PLGE porous microsomes loaded with 5-fluorouracil
[0045] 1) Preparation of PLGE porous microsomes loaded with 5-fluorouracil
[0046] At 15°C, 200 mg of poly(lactide-glycolide-polyethylene glycol ether) copolymer with a molecular weight of 10,000 (the molar ratio of each monomer in PLGE is 60 / 30 / 10, and the molecular weight of PEG is 1000) Dissolve in 10ml dichloromethane (CH 2 Cl 2 ), solution A was obtained after complete dissolution. The solution A was poured into 1 ml of an aqueous solution in which 10 mg of 5-fluorouracil was dissolved, and ultrasonically emulsified for 30 seconds (polymers containing PEG components do not need to add emulsifiers) to form a water-in-oil emulsion. Then this water-in-oil emulsion is slowly added dropwise to 150ml containing the aqueous solution that mass volume concentration is 1% (g / 100ml) polyvinyl alcohol (PVA) to form water-in-oil-in-water emulsion, then continue to stir for 2 hou...
Embodiment 2
[0052] Example 2, preparation and performance of paclitaxel-loaded PGCE porous microsomes
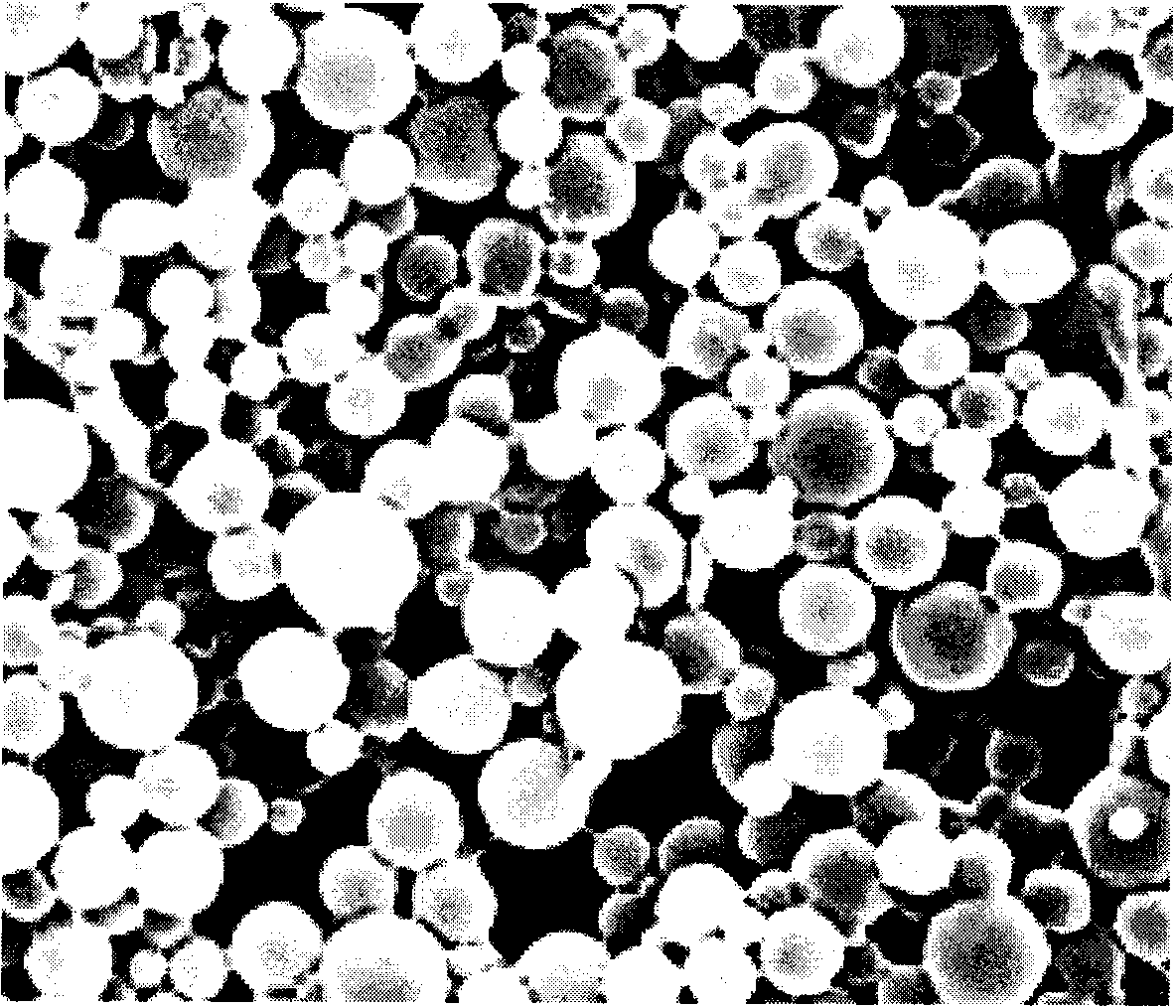
[0053] The preparation method is basically the same as in Example 1. The difference is that the preparation temperature is 23°C, and the carrier used is 200 mg of poly(glycolide-caprolactone-polyethylene glycol ether) copolymer with a molecular weight of 15,000 (the molar ratio of each monomer in PGCE is 30 / 50 in sequence). / 20, the molecular weight of PEG is 2000), the drug is 5 mg paclitaxel, and finally the paclitaxel-loaded PGCE porous microsomes with a particle size of 1 to 8 microns and a porous structure (micropore diameter of 100-1000 nm) are obtained.
[0054] To culture human liver cancer cells, add paclitaxel-loaded PGCE microsomes to the culture medium, and at the same time, apply ultrasonic sound intensity of 0.5W / cm to the cell culture medium 2 After irradiating for 30s, cell apoptosis was measured by fluorescent staining. The results showed that paclitaxel-loaded PGCE 3...
Embodiment 3
[0055] Embodiment 3, preparation and performance of PGCE porous microsomes loaded with heparin
[0056] The preparation method is basically the same as in Example 1. The difference is that the preparation temperature is 20°C, and the carrier used is 180 mg of poly(glycolide-caprolactone-polyethylene glycol ether) copolymer with a molecular weight of 12,000 (the molar ratio of each monomer in PGCE is 30 / 60 in sequence). / 10, the molecular weight of PEG is 1000), the drug is 8 mg heparin, and finally the heparin-loaded PGCE microsomes with a particle size of 1-8 microns and a porous structure (micropore diameter of 100-1000 nm) are obtained.
[0057] The same method as in Example 1 and the results of intravenous infusion with dogs as simulated animals show that the heparin-loaded PGCE microsomes have excellent in vivo ultrasound imaging functions.
[0058] The heparin-loaded PGCE microparticles were made into a suspension and injected into the rat body through the femoral vein ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com