Thiosemicarbazide compound, synthesis method and application to colorimetric detection of mercury ions
A technology of thiosemicarbazide and compounds, which is applied in the fields of analyzing materials through chemical reactions, analyzing materials through observing the influence of chemical indicators, organic chemistry, etc., to achieve the effect of good cation recognition ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
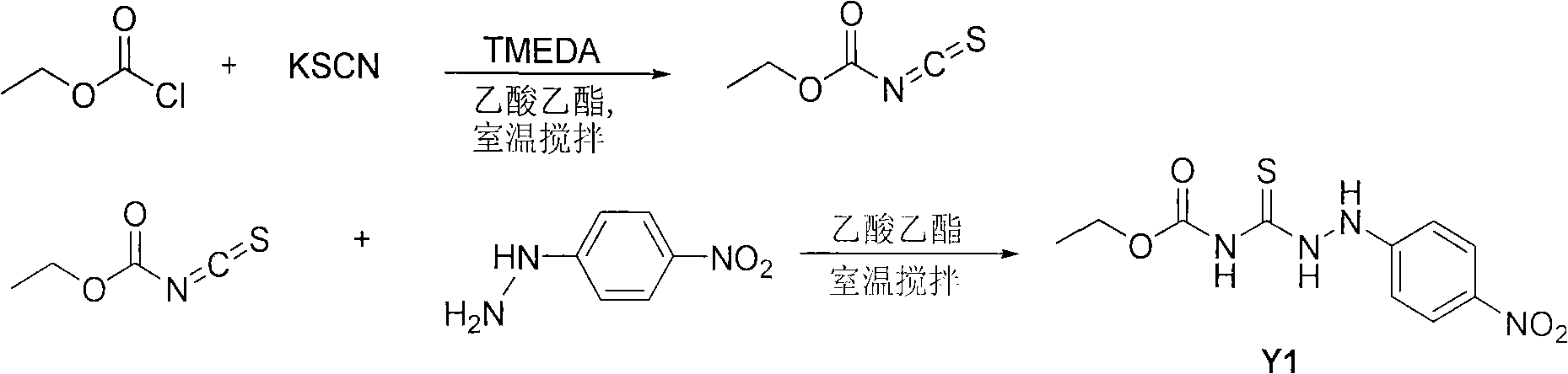
[0038] (1) Synthesis of acceptor 1-nitrophenyl-4-ethoxycarbonylthiosemicarbazide (Y1)
[0039] Dissolve 10mmol of ethyl chloroformate into 20mL of ethyl acetate, add 12mmol of potassium thiocyanate, 0.1mL of N,N,N',N'-tetramethylethylenediamine (TMEDA) as a catalyst, and stir the reaction at room temperature 5 hours. Filter to remove inorganic salts to obtain a solution of the intermediate ethoxycarbonyl isothiocyanate. Add 9.5 mmol of 4-nitrophenylhydrazine to this solution, stir and react at room temperature for 5 hours, and a precipitate is formed. Most of the solvent was distilled off under reduced pressure, and stood at room temperature for 3 hours. The precipitate was filtered and recrystallized with absolute ethanol to obtain crystals of the product Y1. The synthetic route is as follows:
[0040]
[0041] Y1: Yield: 89.7%; m.p.168~170℃; 1 H-NMR (DMSO-d 6 , 400MHz) δ11.32(s, 1H, NH), 11.24(s, 1H, NH), 9.46(s, 1H, NH), 8.08(d, J=9.2, 2H, ArH), 6.79(q, J =7.2, 2H,...
Embodiment 2
[0045] (1) Synthesis of acceptor 1-nitrophenyl-4-benzoylthiosemicarbazide (Y2)
[0046] Dissolve 10mmol of benzoyl chloride in 20mL of dichloromethane, add 12mmol of ammonium thiocyanate, 0.1mL of polyethylene glycol-400 (PEG-400) as a catalyst, and stir at room temperature for 5 hours. Filter to remove inorganic salts to obtain a solution of the intermediate benzoyl isothiocyanate. Add 9.5 mmol of 4-nitrophenylhydrazine to this solution, stir and react at room temperature for 5 hours, and a precipitate is formed. Most of the solvent was evaporated under reduced pressure, and the precipitate was left to stand at room temperature for 3 hours. The precipitate was filtered and recrystallized with anhydrous acetonitrile to obtain crystals of the product Y2. The synthetic route is as follows:
[0047]
[0048] Y2: Yield: 95.7%; m.p.199~201℃; 1 H-NMR (DMSO-d 6 , 400MHz) δ12.02 (s, 2H, NH), 9.77 (s, 1H, NH), 8.11-6.93 (m, 9H, ArH); IR (KBr, cm -1 )v: 3444(mb, N-H), 3310(m, N-...
Embodiment 3
[0052] (1) Synthesis of acceptor 1,3-dinitrophenyl-4-ethoxycarbonylthiosemicarbazide (Y3)
[0053] Dissolve 10mmol of ethyl chloroformate into 20mL of ethyl acetate, add 12mmol of potassium thiocyanate, 0.1mL of N,N,N',N'-tetramethylethylenediamine (TMEDA) as a catalyst, and stir the reaction at room temperature 5 hours. Filter to remove inorganic salts to obtain a solution of the intermediate ethoxycarbonyl isothiocyanate. Add 9.5 mmol of 2,4-dinitrophenylhydrazine to this solution, stir and react at room temperature for 5 hours, and a precipitate is formed. Most of the solvent was evaporated under reduced pressure, and the precipitate was left to stand at room temperature for 3 hours. The precipitate was filtered and recrystallized with absolute ethanol to obtain crystals of the product Y3. The synthetic route is as follows:
[0054]
[0055] Y3: Yield: 96.5%; m.p.208~210°C; 1H NMR (DMSO-d6, 400MHz) δ11.49(s, 2H, NH), 10.44(s, 1H, NH), 8.88(s, 1H, ArH ), 8.37~8.33(m, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com