Method and device for producing thiophene and derivant thereof
A production method and production device technology, which is applied in the field of production methods and devices of thiophene and its derivatives, can solve the problems of heating, sulfur clogged pipes, and cooling to 20°C, etc., to solve the problem of strong odor, sulfur clogging, Achieve cleaning effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] The production of embodiment 1 thiophene
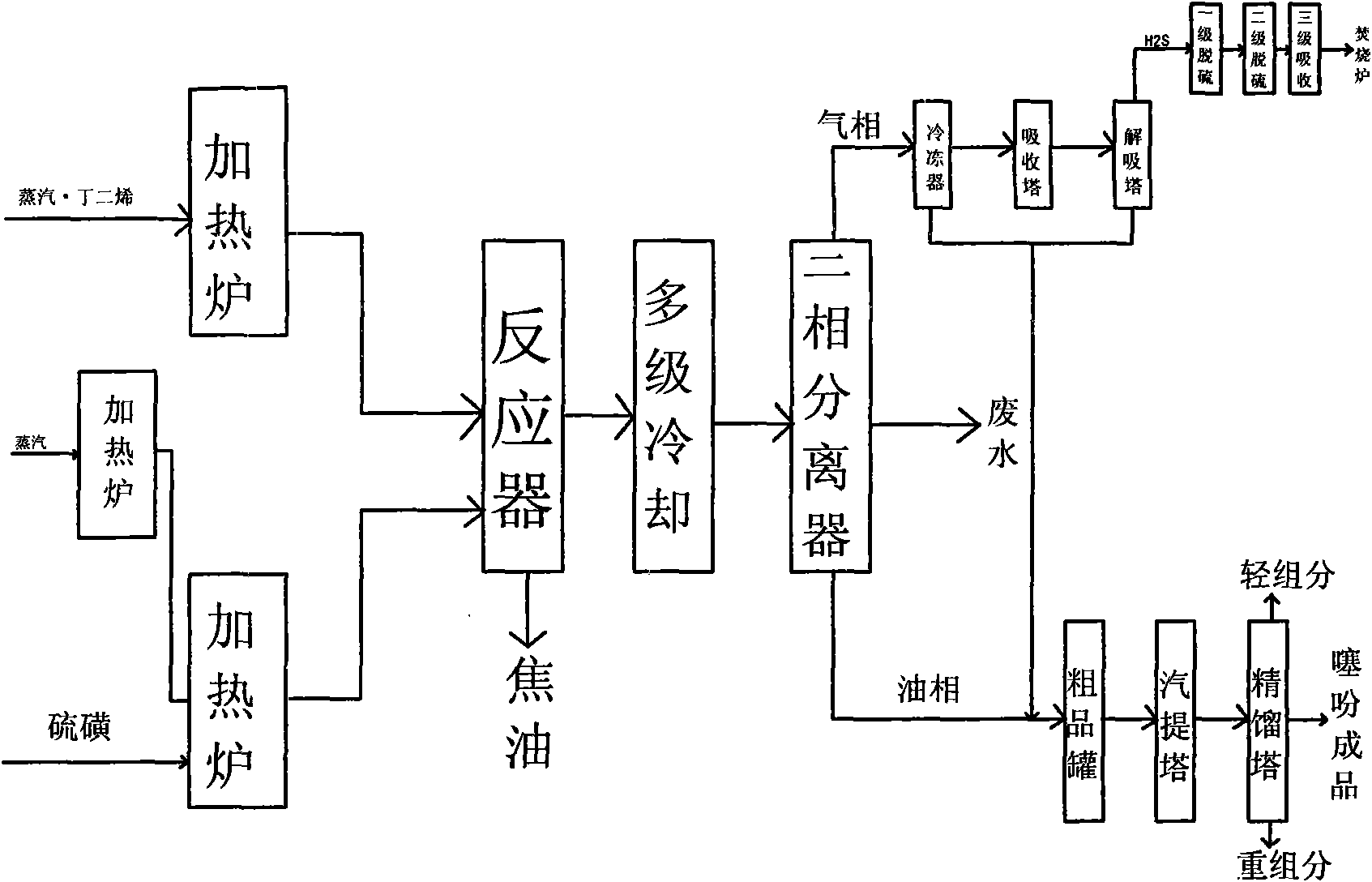
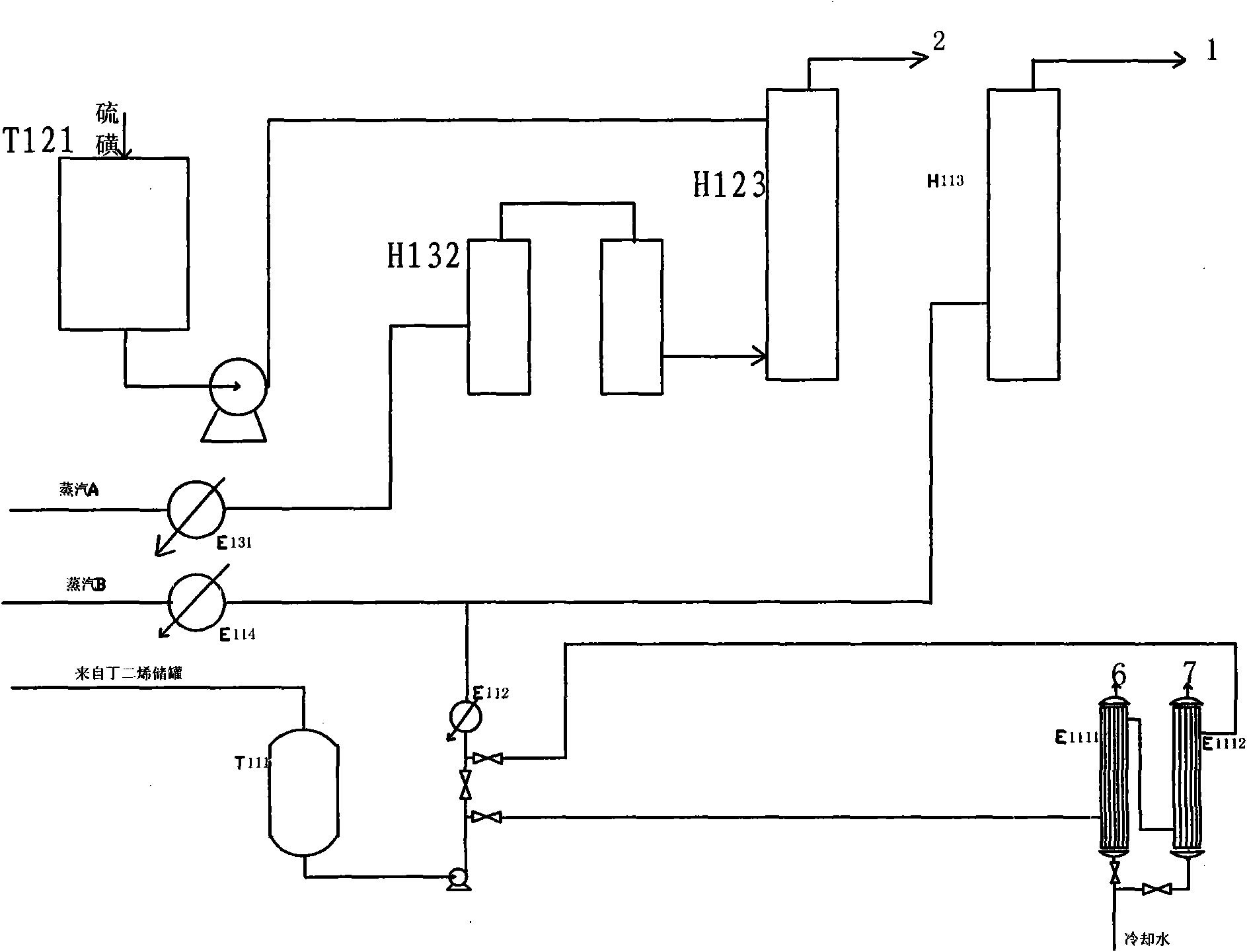
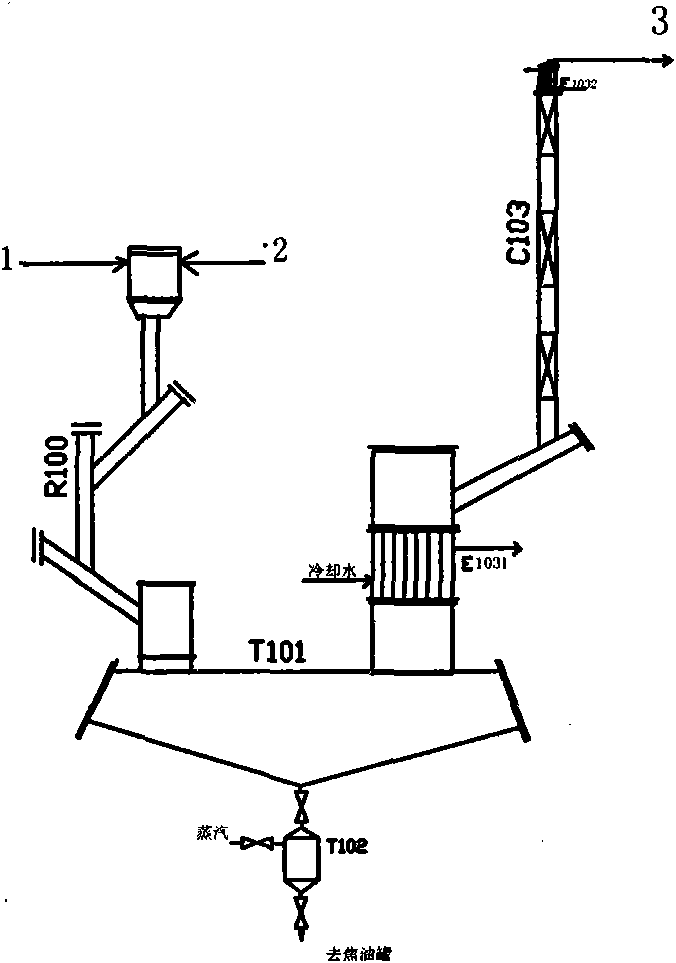
[0086] The butadiene is pumped from the butadiene metering tank T111 to the water heat exchangers E1111 and E1112 with a feed rate of 150Kg / h. After exchanging heat with circulating water, it enters the butadiene vaporizer E112 and is heated to 120°C. The water vapor enters the water B evaporator E114 through the flowmeter, the feed rate is 10Kg / h, and it is heated to 140°C. The butadiene and water B from the vaporizer enter the butadiene heating furnace H113 and are heated to 250°C. The solid sulfur is poured into the sulfur melting tank T121, pumped to the feed inlet of the sulfur heating furnace H123, and the feed rate is 275Kg / h. The water vapor enters the water A vaporizer E131 through the flowmeter, the feed rate is 80Kg / h, and is heated to 140°C, then enters the water heating furnace H132 and is heated to 530°C, and then enters the feeding port below the sulfur heating furnace H123. Water A and sulfur are heated to 540...
Embodiment 2
[0096] The production of embodiment 2 thiophenes
[0097] Process flow and device are with embodiment 1, and its difference is only in:
[0098] Feed amount butadiene 130Kg / h, sulfur 234Kg / h, water A 80Kg / h, water B 10Kg / h. The outlet temperature of the butadiene furnace is 250°C, and the outlet temperature of the sulfur furnace is 545°C. The reactor inlet temperature is 390°C, the outlet temperature is 420°C, the pressure is 0.02MPa (gauge pressure), and the tar kettle outlet temperature is 160°C. Feed ratio sulfur / butadiene 1.52mol, water / butadiene 2.08mol, reaction residence time 1.90s. The output is 2700Kg per day. The unit consumption of butadiene is 1.16 tons, and the yield is 55%. Stop once every 20 days of operation to clean up the tar kettle.
Embodiment 3
[0099] The production of embodiment 3 thiophene
[0100] Process flow and device are with embodiment 1, and its difference is only in:
[0101] Feed amount of butadiene 167Kg / h, sulfur 290Kg / h, water A 90Kg / h, water B 10Kg / h. The outlet temperature of the heating furnace is 250°C for the butadiene furnace and 510°C for the sulfur furnace. The reactor inlet temperature is 375°C, the outlet temperature is 450°C, the pressure is 0.02MPa (gauge pressure), and the tar kettle outlet temperature is 210°C. Feed ratio sulfur / butadiene 1.46mol, water / butadiene 1.8mol, reaction residence time 1.54s. The output is 4600Kg per day. The unit consumption of butadiene is 0.87 tons, and the yield is 74%. However, the temperature of the tar kettle is high, and coking occurs quickly, and it takes only 5 days to shut down for cleaning.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com