Electrolyte solution, preparation method thereof and use thereof
An electrolyte solution and organic solvent technology, applied in the field of lithium batteries, can solve the problems of lithium storage capacity attenuation, reducing active substances, affecting charge and discharge properties, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038]Weigh 1.3g LiF as component A and 25.6g TPFPB as component B in a beaker, add 25ml propylene carbonate (PC) as component D and 25ml dimethyl carbonate (DMC) as component D after drying Part D, and then stirred by a magnetic stirrer for 1 hour to become a transparent electrolyte solution. Weigh 0.485 g of LiBOB as component C, add it into the previously prepared electrolyte solution, and stir for 1 hour with a magnetic stirrer. Ready to use. All the above operations were performed in an atmosphere filled with argon.
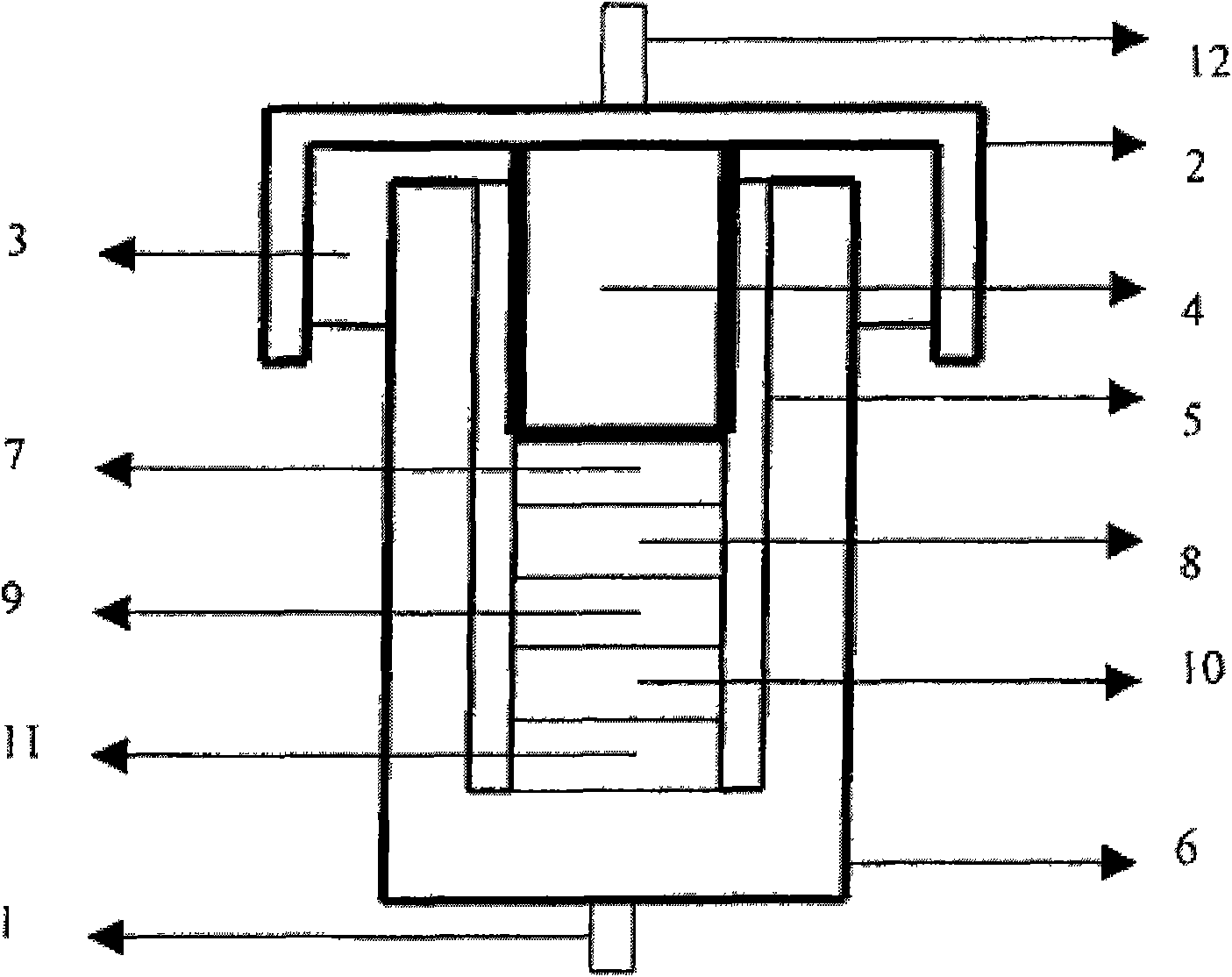
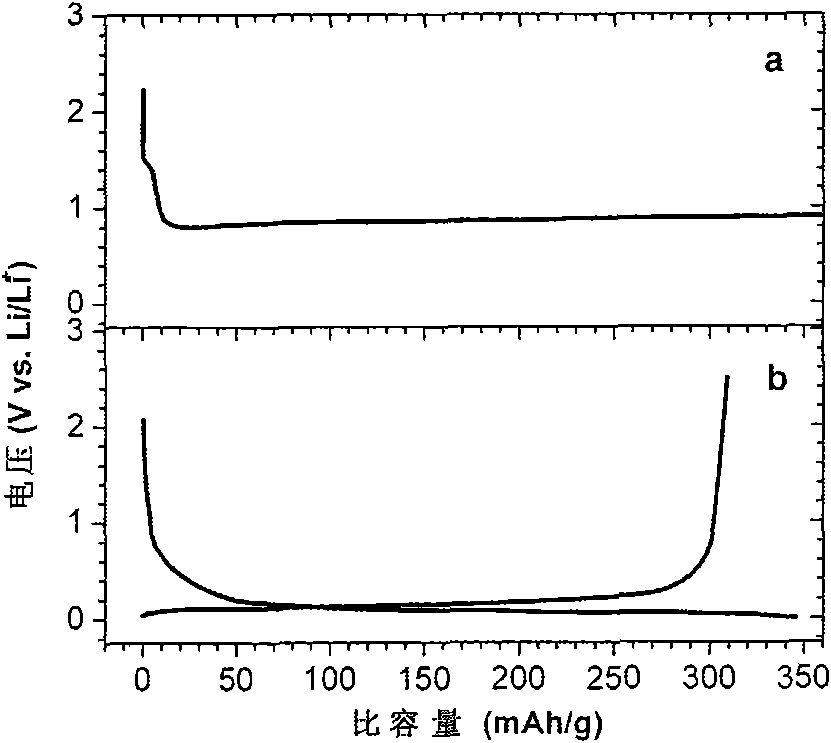
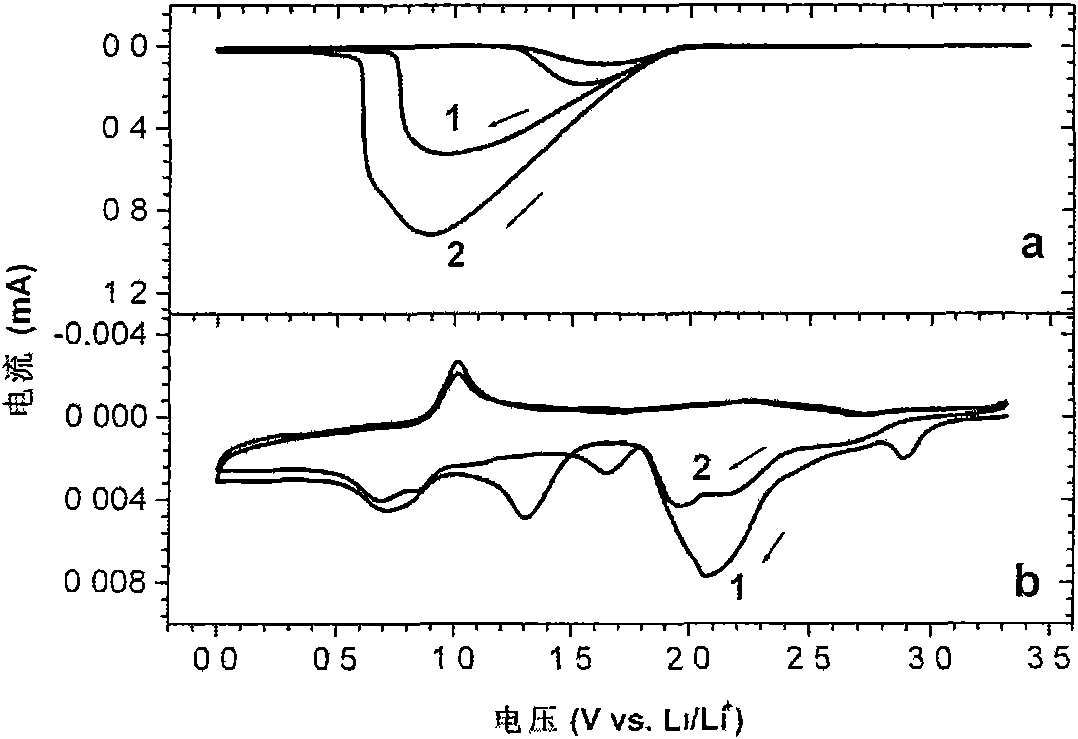
[0039] The electrolyte was added dropwise to a glass conductivity cell with platinum electrodes at both ends, and its conductivity was measured in the range of 5Hz-13MHz using an HP4192 impedance spectrometer, and the measured conductivity at 25°C was 3.2ms / cm. Used in conjunction with GDW6005 high and low temperature test chamber to measure the conductivity of samples at different temperatures. Using CHI627C electrochemical workstation, with the method o...
Embodiment 2
[0045] Weigh 1.3g LiF as component A and 25.6g TPFPB as component B in a beaker, add 25ml ethylene carbonate (EC) as component D and 25ml diethylcarbonate (DEC) as component D after drying Part D, and then stirred by a magnetic stirrer for 1 hour to become a transparent electrolyte solution. Weigh 0.388 g of LiBOB as component C, add it into the previously prepared electrolyte solution, and stir for 1 hour with a magnetic stirrer. Ready to use. All the above operations were performed in an atmosphere filled with argon.
[0046] The electrolyte was dropped into a glass conductivity cell with platinum electrodes at both ends, and its conductivity was measured in the range of 5 Hz-13 MHz using an HP4192 impedance spectrometer, and the measured conductivity at 25°C was 3.1 ms / cm. Used in conjunction with GDW6005 high and low temperature test chamber to measure the conductivity of samples at different temperatures. Using CHI627C electrochemical workstation, with the method of cy...
Embodiment 3
[0049] Weigh 1.3g LiF as component A and 25.6g TPFPB as component B in a beaker, add 25ml propylene carbonate (PC) as component D and 25ml ethylene carbonate (EC) as component D after drying , and then stirred by a magnetic stirrer for 1 hour to become a transparent electrolyte solution. Weigh 0.243 g of LiBOB as component C, add it into the previously prepared electrolyte solution, and stir for 1 hour with a magnetic stirrer. Ready to use. All the above operations were performed in an atmosphere filled with argon.
[0050] The electrolyte was dropped into a glass conductivity cell with platinum electrodes at both ends, and its conductivity was measured in the range of 5 Hz-13 MHz using an HP4192 impedance spectrometer, and the measured conductivity at 25°C was 3.1 ms / cm. Used in conjunction with GDW6005 high and low temperature test chamber to measure the conductivity of samples at different temperatures. Using CHI627C electrochemical workstation, with the method of cyclic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Current density | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com