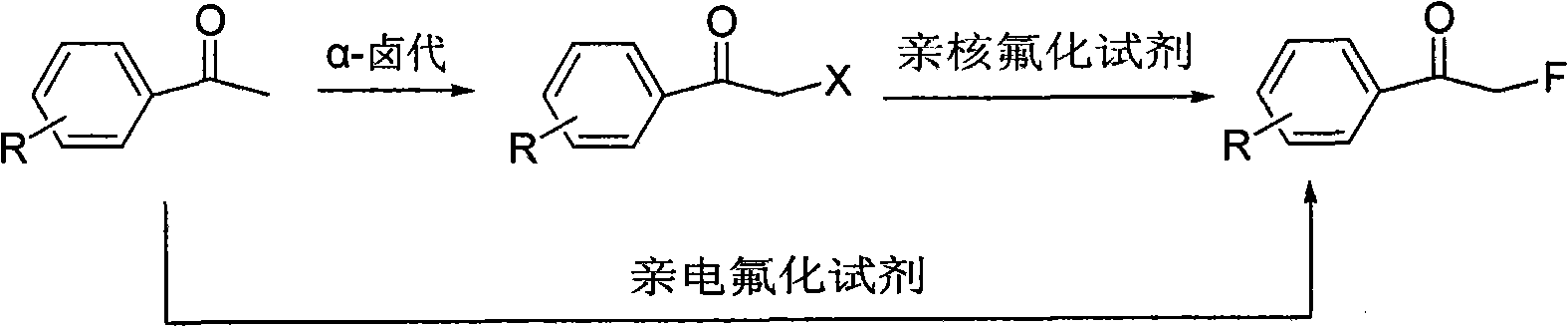
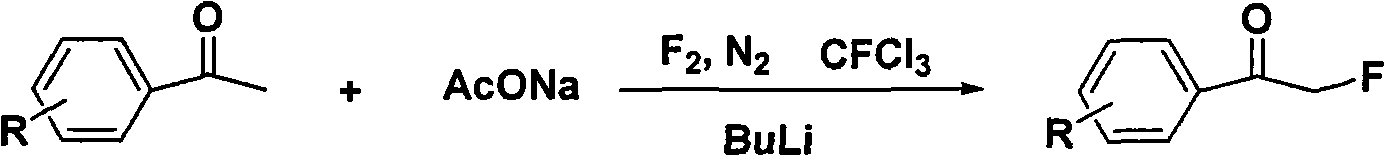Method for directly preparing alpha-fluoro acetophenone by acetophenone one-pot method
A technology for substituting acetophenone and acetophenone, which is applied in the field of preparation of fine chemical products, can solve the problems of difficulty in controlling electrophilic fluorination reagents, low yield, expensive use, etc., and achieves reduction of synthesis cost, simple method, Efficiently obtained results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of α-fluoro-p-methylacetophenone from p-methylacetophenone
[0037]Take 1mmol choline chloride and 1mmol TsOH in a round bottom flask, heat to form a eutectic, and slowly cool to room temperature. Add 3ml of acetonitrile, add 2mmol of p-methylacetophenone, slowly add 1.1mmol of DCDMH, react for 3 hours, and continue to stir for 1 hour. After adding 5 mmol of zinc fluoride, 6 mmol of tetramethylammonium fluoride was added in batches, and the reaction was continued for 8 hours at 50° C. After filtering, the filtrate was rotary evaporated to remove the solvent, then added 20ml of 5% dilute hydrochloric acid, extracted with 20ml of dichloromethane, and washed twice with 20ml of water until neutral. MgSO 4 After drying and rotary evaporation to remove the solvent, a crude product was obtained, which was separated and purified by column chromatography to obtain the product α-fluoro-p-methylacetophenone. yield and product 1 The H NMR data are shown in Table 1. ...
Embodiment 2
[0039] Preparation of α-fluoroacetophenone dimethyl ketal from acetophenone
[0040] Take 1mmol of choline chloride and 1mmol of zinc chloride in a round bottom flask, heat to form a eutectic, and slowly cool to room temperature. Add 2 mmol of acetophenone, slowly add 2.0 mmol of NCS, react for 3 hours, and continue stirring for 1 hour. After adding 3 mmol of potassium fluoride, 6 mmol of tetrabutylammonium fluoride was added in batches, and the reaction was continued at 80° C. for 5 hours. After filtering, the filtrate was rotary evaporated to remove the solvent, then added 20ml of 5% dilute hydrochloric acid, extracted with 20ml of dichloromethane, and washed twice with 20ml of water until neutral. MgSO 4 After drying and rotary evaporation to remove the solvent, a crude product was obtained, which was separated and purified by column chromatography to obtain the product α-fluoroacetophenone. yield and product 1 The H NMR data are shown in Table 1.
Embodiment 3
[0042] Preparation of α-fluoro-p-bromoacetophenone from p-bromoethanone
[0043] Take 1mmol of tetrabutylammonium bromide and 1mmol of p-toluenesulfonic acid in a round-bottomed flask, heat to form a eutectic, and slowly cool to room temperature. Add 3 ml of DMF, add 2 mmol of p-bromoacetophenone, slowly add 1.2 mmol of DBDMH, react for 3 hours, and continue stirring for 1 hour. After adding 2 mmol of CsF first, 6 mmol of tetramethylammonium fluoride was added in batches, and the reaction was continued for 4 hours at 100° C. After filtering, the filtrate was rotary evaporated to remove the solvent, then added 20ml of 5% dilute hydrochloric acid, extracted with 20ml of dichloromethane, and washed twice with 20ml of water until neutral. MgSO 4 After drying and rotary evaporation to remove the solvent, a crude product was obtained, which was separated and purified by column chromatography to obtain the product α-fluoro-p-bromoacetophenone. yield and product 1 The H NMR data a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com