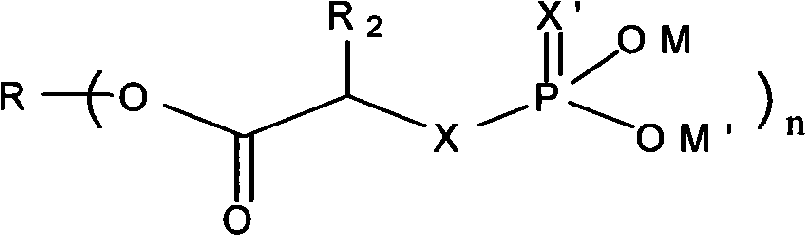
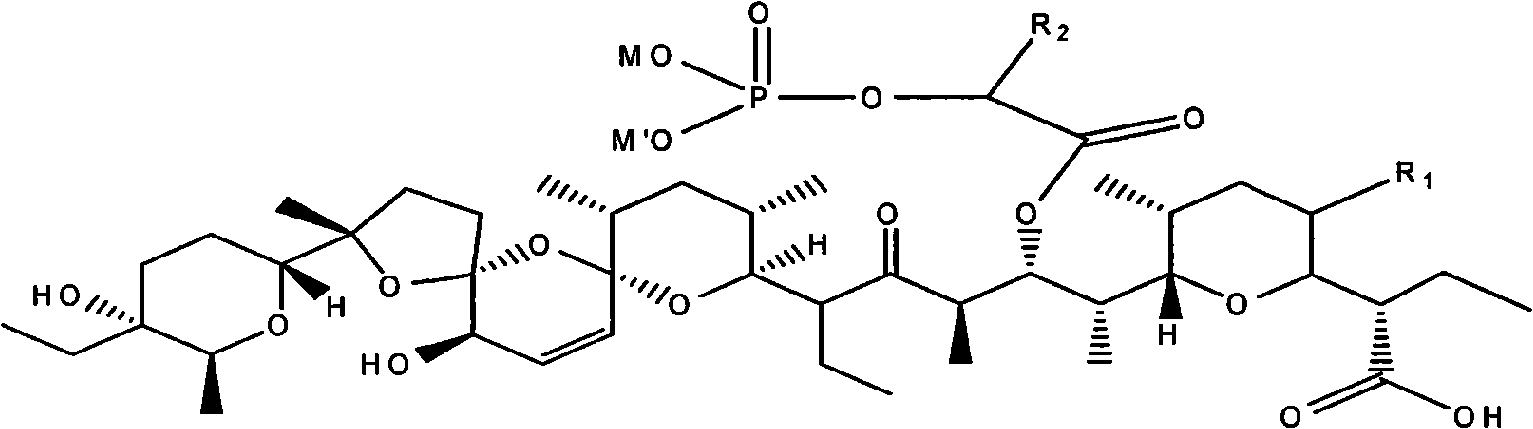
Phosphoryl carboxylic acid salinomycin ester derivative and preparing method thereof
A technology of risamycin ester and phosphoryl carboxylic acid, which is applied in the field of phosphoryl methyl salinomycin ether derivatives and its preparation, can solve the problems of easy local pain, poor water solubility, and influence on practical application, and increase the Good compliance, good stability, fast onset effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Synthesis of Salinomycin 9-Chloroacetate
[0051]
[0052] Dissolve salinomycin (20mg, 0.027mmol) in pyridine (1ml), add chloroacetic anhydride (9.1mg, 0.053mmol) dropwise in an ice bath under magnetic stirring, protect with nitrogen, keep anhydrous environment, and dropwise add Finally, the ice bath was removed, the reaction solution was gradually raised to room temperature, and stirred for 3 hours under the protection of argon. After the spot reaction was complete, ethyl acetate and water were added, extracted with ethyl acetate, the organic phases were combined, and washed 2-3 times with water. , the organic phase was dried, filtered, and concentrated to obtain salinomycin 9-chloroacetate.
[0053] 1H-NMR (D6-DMSO-D2O): δ0.96 (3CH3, t, 9H), 1.06 (4CH3, d, 12H), 1.16 (CH3, d, 3H), 1.21 (CH3, d, 3H), 1.31 (CH3, s, 3H), 1.44 (CH2, m, 2H), 1.56 (CH2, m, 2H), 1.57 (CH2, m, 2H), 1.64, 1.39 (CH2, m, 2H), 1.68, 1.43 ( CH2, m, 2H), 1.76 (CH, m, H), 1.84, 1.59 (CH2, m, 2...
Embodiment 2
[0055] Synthesis of 9-(phosphorylthio)acetate salinomycin ester disodium
[0056]
[0057] In a three-necked flask, add sodium thiophosphate (7.2mg, 0.02mmol), 9-chloroacetic acid salinomycin ester (18.21mg, 0.022mmol) and 0.4ml of distilled water at one time, stir, and under ice water cooling, in the flask The temperature naturally drops to about 15°C, while adding 0.12ml of dimethyl sulfoxide (DMSO) dropwise, the reaction temperature rises gradually, and does not exceed 20°C. After the addition is completed, continue to stir until the reaction is complete to obtain a product solution. Add acetone (the amount of acetone is 5 times the volume of the mother liquor), stir, add anhydrous sodium sulfate to it and dry for 2 hours, filter, pull the mother liquor to dry at room temperature to remove acetone, add ether to the residue to precipitate the product, wash with ether, and dry After that, 9-(phosphorylthio) acetate disodium salinomycin was obtained.
[0058] 1 H-NMR (D6-...
Embodiment 3
[0060] Synthesis of Disodium 9-(Phosphoryloxy) Salinomycin Ester Acetate
[0061]
[0062] In a three-necked flask, add sodium phosphate (7.6mg, 0.02mmol), 9-chloroacetic acid salinomycin ester (18.21mg, 0.022mmol) and 0.4ml of distilled water at one time, stir, and under ice water cooling, the temperature in the flask is natural Decrease to about 15°C, dropwise add 0.12ml of dimethyl sulfoxide (DMSO), the reaction temperature rises gradually, not exceeding 20°C, after the addition is complete, continue to stir until the reaction is complete to obtain a product solution, filter the reaction solution, add acetone to the mother liquor (the amount of acetone is 5 times the volume of the mother liquor), stir, add anhydrous sodium sulfate to it and dry for 2 hours, filter, pull the mother liquor at room temperature to remove acetone, add ether to the residue to precipitate the product, wash with ether, and dry to obtain 9-(phosphoryloxy) salinomycin disodium acetate, 1 molecule ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com