Conformal ultrasonic system for enforcing medicaments to permeate blood brain barrier
A blood-brain barrier and ultrasound system technology, applied in the field of medical devices, can solve problems such as no research reports, no ultrasound array and control parameters involved, and no accurate focusing of low-frequency ultrasound through the skull, so as to avoid damage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1, promoting the composition and mode of action of drugs through the blood-brain barrier system
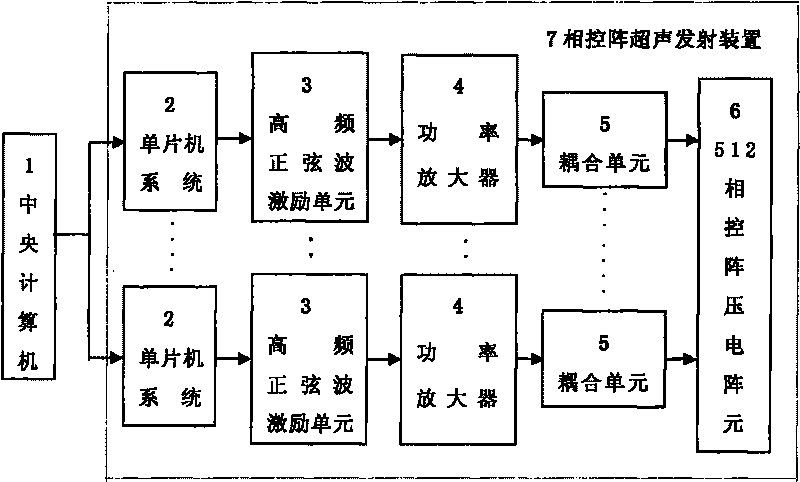
[0029] Such as figure 1 As shown, the conformal ultrasound system for promoting drugs to pass through the blood-brain barrier is mainly composed of a central computer 1 and a phased array ultrasound emitting device 7. The phased array ultrasound transmitting device 7 is composed of 512 ultrasound emitting units, and each ultrasound emitting The unit is composed of a single-chip microcomputer system 2, a high-frequency excitation unit 3, a power amplifier 4, a coupling unit 5 and a phased array piezoelectric array element 6. The single-chip system 2 controls the high-frequency excitation unit 3 to synthesize high-frequency sine waves or high-frequency square waves. High-frequency sine waves or high-frequency square waves can be generated by digital frequency synthesizers, or by LC self-excited oscillation circuits, and then passed through power amplifiers. 4 Ampl...
Embodiment 2
[0045] Embodiment 2, the use method that promotes medicine to pass through the blood-brain barrier system
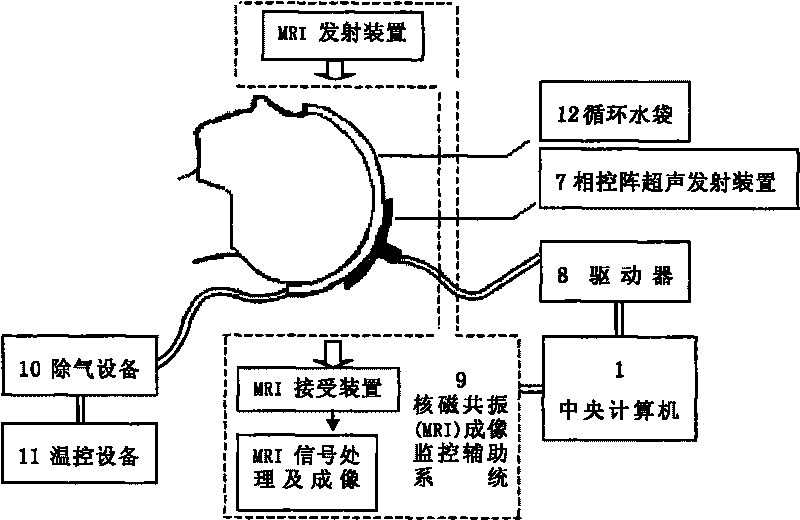
[0046] First let the patient lie flat on a horizontally movable bed with a cap-shaped water bag on his head, and then send the patient into the instrument treatment area.
[0047] When the patient is lying flat, start the nuclear magnetic resonance emission device in the nuclear magnetic resonance imaging auxiliary monitoring system 9, and the brain function image is received by the nuclear magnetic resonance receiving device, and the brain function information is transmitted to the central through the nuclear magnetic resonance signal processing and imaging unit. computer.
[0048] The doctor checks the location of the lesion on the computer, and clicks the mouse to determine the spatial position of the lesion.
[0049] The driver 8 is used to move the phased array ultrasonic emitting device 7 to emit ultrasonic waves to the ideal treatment site; then the central compu...
Embodiment 3
[0054] Embodiment 3, promoting the therapeutic effect of medicine through the blood-brain barrier system
[0055] The effect display is divided into two parts: in vitro experiment and in vivo experiment.
[0056] The establishment of the blood-brain barrier model in vitro was achieved by co-culturing rabbit brain microvascular endothelial cells and astrocytes. Studies have proved that under specific ultrasonic parameters, ultrasonic waves can significantly increase the permeability of cell membranes, allowing macromolecular gene drugs to enter cells and express them. The transfection rate is 40%. The intensity of gene expression in cells and the amount of specific control parameters closely related, leading to the conclusion that it can be safely used for gene delivery under the precise effect scale control system, from Figure 5 It can be seen that through the scanning electron microscope, the repairable micropores on the S180 cell membrane caused by the action of ultrasound...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pulse width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com