Homodisperse ferrite magnetic manoparticles and preparation method thereof
A technology of magnetic nanoparticles and uniform dispersion, applied in the direction of magnetism of inorganic materials, can solve the problems of low crystallinity, high energy consumption, impurities in products, etc., and achieve the effect of saving production energy consumption, reducing processing temperature and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
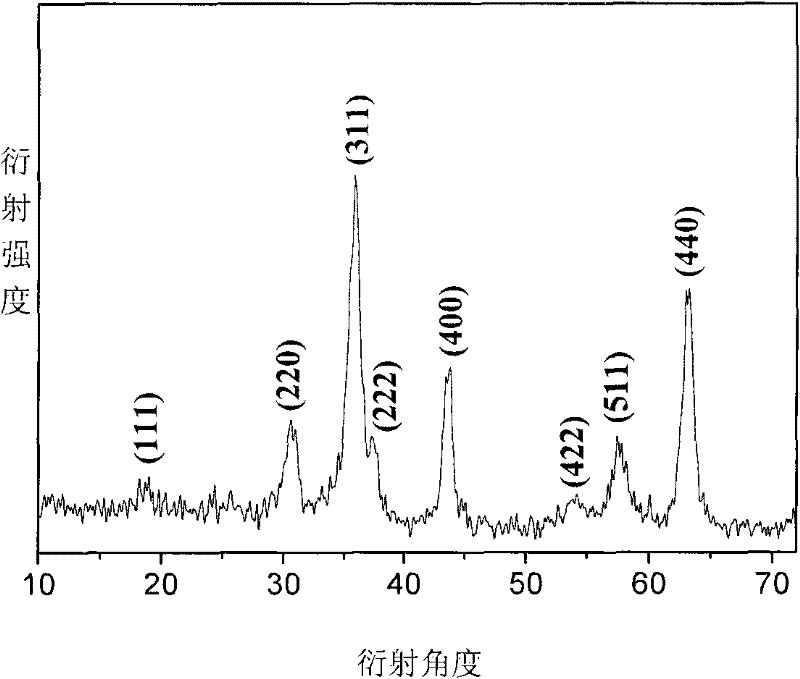
Image
Examples
Embodiment 1
[0023] 1.7441g Ni(NO 3 ) 2 ·6H 2 O and 4.8462g Fe(NO 3 ) 3 9H 2 O was added to 30ml deionized water to prepare a mixed salt solution, in which Ni 2+ The molar concentration is 0.2mol L -1 , Fe 3+ The molar concentration is 0.4mol L -1 30 milliliters of mixed alkaline solutions are prepared with 1.152g sodium hydroxide and 2.544g sodium carbonate, wherein the molar concentration of sodium hydroxide is 0.96mol L -1 , the concentration of sodium carbonate is 0.8mol L -1 .
[0024] The prepared salt solution and alkali solution were quickly poured into the fully back-mixed liquid membrane reactor at room temperature, and vigorously rotated and stirred for 3 minutes at a speed of 2000 rpm. The obtained suspension was centrifuged and dehydrated (centrifugal speed 4000 rpm), then washed with deionized water, and the operation was repeated 3 times to obtain hydroxide crystal nuclei;
[0025] Dissolve 3.24g of glucose into 30ml of deionized water to prepare a solution, mix t...
Embodiment 2
[0028] 2.6162g Ni(NO 3 )2 ·6H 2 O and 7.2693g Fe(NO 3 ) 3 9H 2 O was added to 30ml deionized water to prepare a mixed salt solution, in which Ni 2+ The molar concentration is 0.3mol L -1 , Fe 3+ The molar concentration is 0.6mol L -1 30 milliliters of mixed alkaline solutions are prepared with 1.728g sodium hydroxide and 3.816g sodium carbonate, wherein the sodium hydroxide molar concentration is 1.44mol L -1 , the concentration of sodium carbonate is 1.2mol L -1 .
[0029] The prepared salt solution and alkali solution were quickly poured into the fully back-mixed liquid membrane reactor at room temperature, and vigorously rotated and stirred for 2 minutes at a speed of 2500 rpm. The obtained suspension was centrifuged and dehydrated (centrifugal speed 4500 rpm), washed with deionized water, and the operation was repeated 3 times to obtain hydroxide crystal nuclei;
[0030] Dissolve 3.24g of glucose into 30ml of deionized water to prepare a solution, mix the precipi...
Embodiment 3
[0033] 0.8721g Ni(NO 3 ) 2 ·6H 2 O, 0.8922g Zn(NO 3 ) 2 ·6H 2 O and 4.8462g Fe(NO 3 ) 3 9H 2 O was added to 30ml deionized water to prepare a mixed salt solution, in which Ni 2+ The molar concentration is 0.1mol L -1 , Zn 2+ The molar concentration is 0.1molL -1 , Fe 3+ The molar concentration is 0.4mol L -1 30 milliliters of mixed alkaline solutions are prepared with 1.152g sodium hydroxide and 2.544g sodium carbonate, wherein the molar concentration of sodium hydroxide is 0.96mol L -1 , the concentration of sodium carbonate is 0.8mol L -1 .
[0034] The prepared salt solution and alkali solution were quickly poured into the fully back-mixed liquid membrane reactor at room temperature, and vigorously rotated and stirred for 2 minutes at a speed of 2500 rpm. The obtained suspension was dehydrated by centrifugation (centrifugal speed 4500 rpm), and then washed with deionized water, and the operation was repeated 3 times to obtain hydroxide crystal nuclei;
[003...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com