Method for preparing macroporous polymer fixed quinonoid compound
A technology of quinone compounds and polymers, which is applied in the field of preparation of macroporous polymers to immobilize quinone compounds, can solve problems such as secondary pollution, accelerated anaerobic biotransformation of refractory organic matter, large specific surface area, etc., to solve secondary pollution, Good catalytic performance, the effect of solving technical bottlenecks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
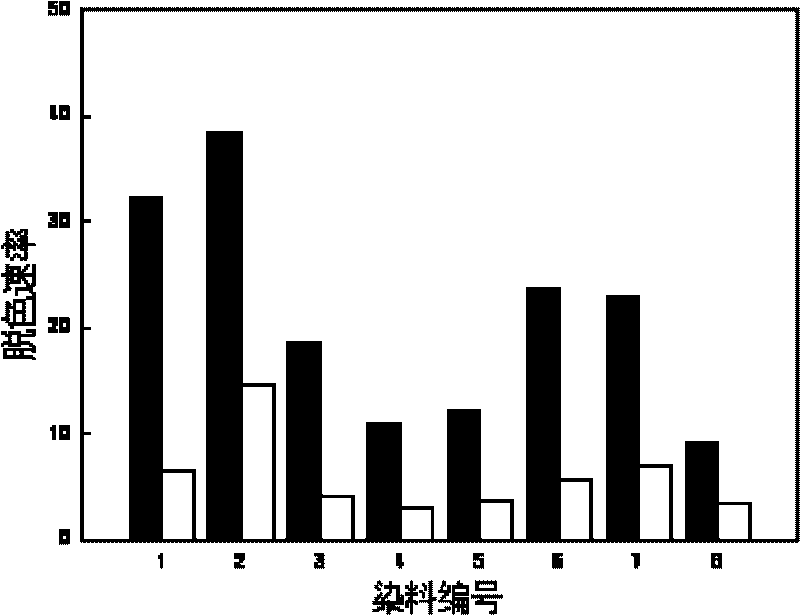
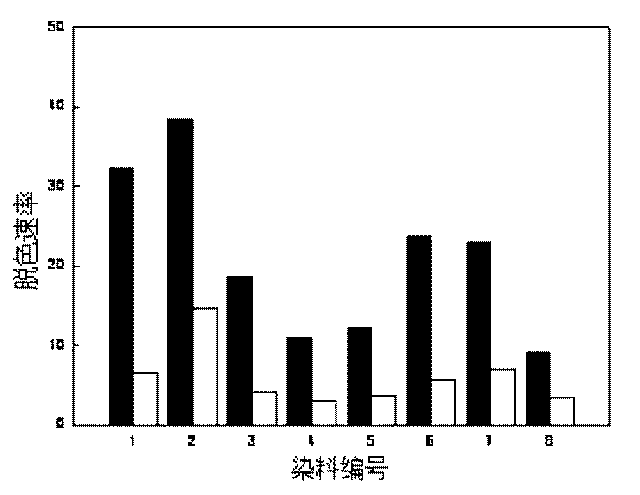
[0019] At room temperature, in 50mL of 2mmol / L sodium hydroxide solution, add 24g of diethylenetriamine and 5 pieces of washed and dried polyvinyl alcohol foam (about 1cm in size) 3 ), after stirring and reacting for 3 hours, rinse with distilled water and dry. In a 500mL flask with a stirrer, add 5 pieces of aminated polyurethane foam and 100mL of 2mmol / L NaOH solution; dissolve 0.6g of anthraquinone-2-sulfonyl chloride in 25mL of dichloromethane, and then This was added over 30 minutes and stirring was continued for 1 hour before removal. Wash with distilled water and dry to obtain polyvinyl alcohol foam containing quinone compound. The amount of quinone contained in 1 gram of polyvinyl alcohol foam block prepared by this method is 0.2 mmol. The polyvinyl alcohol foam containing quinone compounds can promote the anaerobic biotransformation of azo dyes, nitroaromatics and chlorinated aromatics, and its catalytic performance remains basically unchanged after repeated use for...
Embodiment 2
[0021] At room temperature, in 50mL of 2mmol / L sodium hydroxide solution, add 47.85g of diethylenetriamine and 5 pieces of washed and dried 0.68g polyurethane foam containing polyvinyl alcohol (the size is about 1cm 3 ), after stirring and reacting for 3 hours, rinse with distilled water and dry. In a 500mL flask with a stirrer, add 5 pieces of aminated polyurethane foam and 100mL of 2mmol / L NaOH solution; dissolve 0.6g of anthraquinone-2-sulfonyl chloride in 25mL of dichloromethane, and then This was added over 30 minutes and stirring was continued for 1 hour before removal. Wash with distilled water and dry to obtain polyurethane foam containing quinone compound. The amount of quinone contained in 1 gram of polyurethane foam block prepared by this method is 0.097 mmol. The polyurethane foam containing quinone compounds can promote the anaerobic biotransformation of azo dyes, nitroaromatics and chlorinated aromatics, and its catalytic performance remains basically unchanged...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com