Transdermal nitrendipine adhesive patch and preparation method thereof
A technology of dipine patch and nitrendipine, which is applied in the direction of pharmaceutical formulation, sheet delivery, drug combination, etc., can solve the problems of unfavorable application, poor mutual binding force between the drug layer and silicon paper, and difficulty in mixing uniformly, so as to facilitate the application , shorten the production time, good overall effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
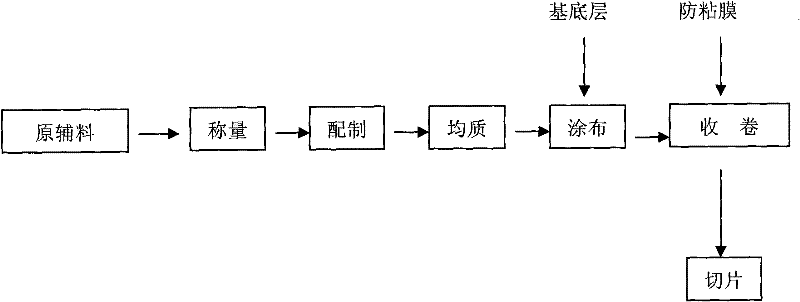
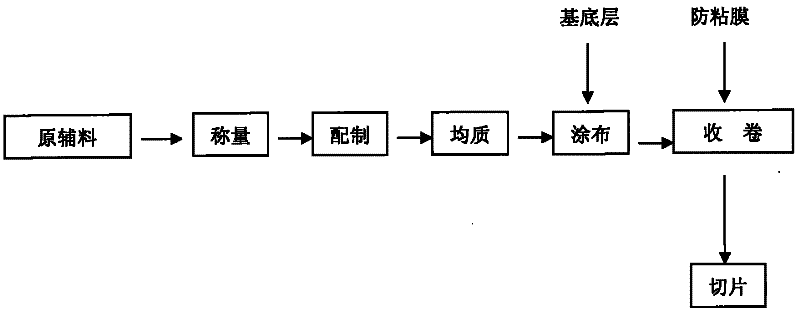
[0035] (1) Take by weighing dehydrated alcohol 30g and dissolve polyacrylic acid resin IV70g, add 200g pressure-sensitive adhesive and stir evenly;
[0036] (2) Take by weighing 10g azone, 30g propylene glycol and 30g dehydrated alcohol join in the 50g nitrendipine and stir;
[0037] (3) Add to item (1), stir for 10 minutes, and homogenize twice at 7mpa with a homogenizer;
[0038] (4) Set the drying temperature of the coating machine to 60°C, control the thickness to 35um, perform coating, and then cover the medical elastic cloth;
[0039] (5) Cut into 4*6cm with a slicer 2 .
[0040] According to the method provided by the national drug standard WS 1-(X-343)-2003Z issued by the State Food and Drug Administration, the content standard range: 80.0%-120.0%, actual detection: 100.4%, content uniformity standard: Less than 15%, actual detection: 9.4%.
Embodiment 2
[0042] (1) Weigh 40g of absolute ethanol to dissolve 87.5g of polyacrylic acid resin IV, add 248.5g of pressure-sensitive adhesive and stir evenly;
[0043] (2) Take by weighing 15g azone, 45g propylene glycol and 40g dehydrated alcohol join in the 50g nitrendipine and stir;
[0044] (3) Add item (2) to item (1), stir for 15 minutes, and homogenize 3 times at 12mpa with a homogenizer;
[0045] (4) Set the drying temperature of the coating machine to 75°C, control the thickness to 45um, perform coating, and then cover the medical elastic cloth;
[0046] (5) Cut into 4*6cm with a slicer 2 .
[0047] The test results are as follows: content standard range: 80.0%-120.0%, actual test: 100.2%, content uniformity standard: less than 15%, actual test: 14.2%.
Embodiment 3
[0049] (1) Take by weighing dehydrated alcohol 50g and dissolve polyacrylic acid resin IV95g, add 350g pressure-sensitive adhesive and stir evenly;
[0050] (2) Take by weighing 25g azone, 60g propylene glycol and 60g dehydrated alcohol join in the 50g nitrendipine and stir;
[0051] (3) Add item (2) to item (1), stir for 25 minutes, and homogenize 4 times at 14mpa with a homogenizer;
[0052] (4) Set the drying temperature of the coating machine to 82°C, control the thickness to 65um, perform coating, and then cover the medical elastic cloth;
[0053] (5) Cut into 4*6cm with a slicer 2 .
[0054] The test results are as follows: content standard range: 80.0%-120.0%, actual test: 99.5%, content uniformity standard: less than 15%, actual test: 10.2%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com