Biquaternary ammonium salt and bisulfonate surfactant and synthesis method thereof
A technology of surfactants and bissulfonates, applied in the preparation of sulfonates, chemical instruments and methods, organic chemistry, etc., can solve the problems of late Gemini surfactants and limited types of Gemini surfactants, and achieve a synthesis method. Simple, excellent surface activity, easy to operate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The preparation of embodiment 1 TM-12
[0034] (1) Synthesis of N, N'-(di-p-benzenesulfonic acid)ethylenediamine
[0035] In a 250mL three-necked flask placed in a constant temperature water bath and equipped with a stirrer, a thermometer, and a reflux condenser, add 4 g (0.0231 mol) of p-aminobenzenesulfonic acid and an aqueous sodium hydroxide solution of ethanol, and slowly add 1,2-bis Bromoethane 2.1604g (0.0115mol) solution, adjust the pH value at 8-10, stir and reflux at a water bath temperature of 65-85°C, and react for 35-40 hours. The reaction solution was slowly evaporated to dryness at 50° C. to obtain 4.17 g of white solid (yield about 97%).
[0036] (2) Synthesis of N, N'-(sodium dialkyl di-p-benzenesulfonate) ethylenediamine
[0037] Add 4g (0.01074mol) of the intermediate to a three-necked flask equipped with a stirrer and a thermometer placed in a constant temperature water bath, then add 100mL of distilled water / ethanol (v:v=2:3) mixed solvent to diss...
Embodiment 2
[0040] The preparation of embodiment 2 TM-10
[0041] (1) Synthesize N, N'-(di-p-benzenesulfonic acid) ethylenediamine and N, N'-(sodium dialkyldi-p-benzenesulfonate) respectively by the method of Example 1 (1), (2) Ethylenediamine.
[0042] (2) Synthesis of TM-10
[0043] In a 250mL three-necked flask placed in a constant temperature water bath and equipped with a stirrer and a reflux condenser, add 4 g (0.0053 mol) of the intermediate N, N'-(sodium dialkyldi-p-benzenesulfonate) ethylenediamine, and then add 50mL of distilled water / ethanol (v:v=2:3) mixed solvent, stir to dissolve, slowly add bromoethane 1.2510g (0.01148mol) dropwise, adjust the pH value to about 8, in the water bath temperature is 30 ~ 50 ℃ React for 7 to 10 hours. After the reaction, a precipitate precipitated out, cooled overnight, filtered with suction, and dried at 50°C to obtain a white solid powder which is TM-10. Weighed 3.9908g (76% yield).
Embodiment 3
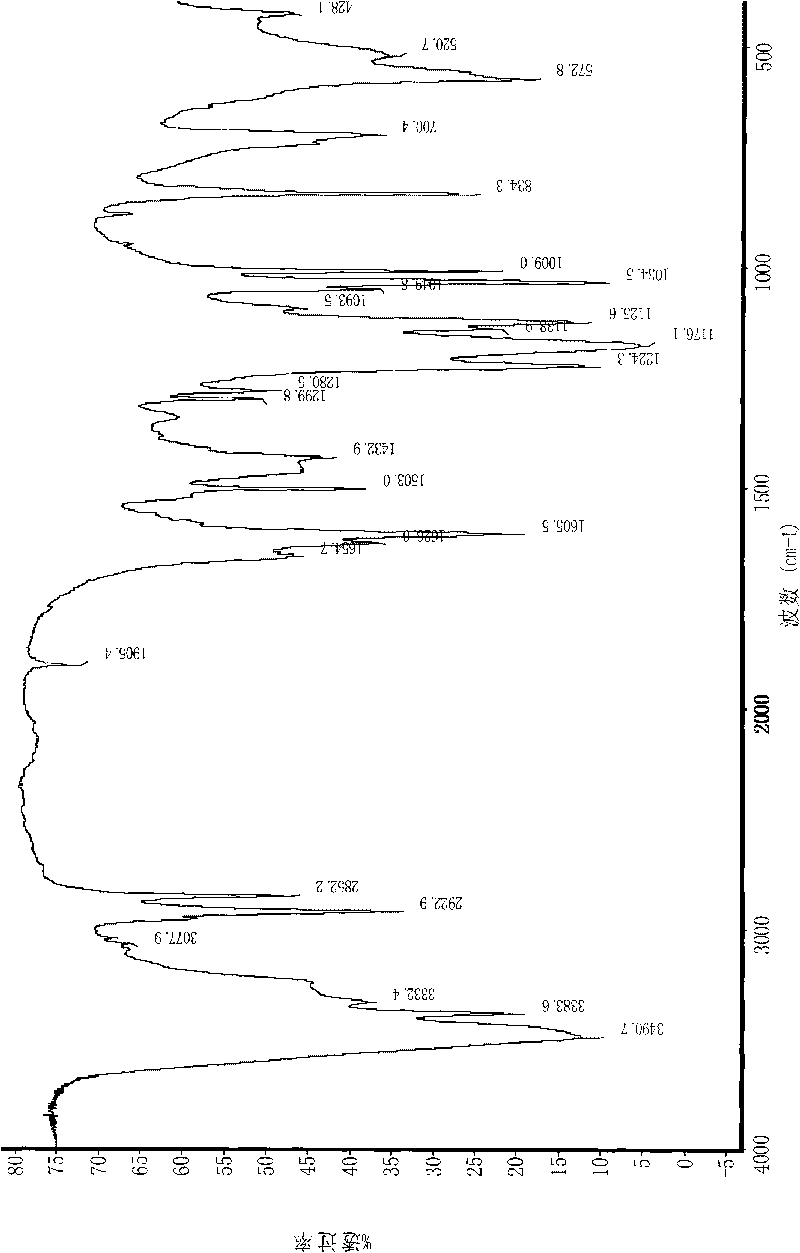
[0044] The infrared spectrum of embodiment 3 target product TM surfactant
[0045] The IR spectrogram of the target substance TM-12 obtained by embodiment 1 is shown in figure 1 .
[0046] The attribution analysis of each characteristic band in the spectrum is as follows: 3490.7, 3383.6, 3332.4cm -1 It is O-H stretching vibration on amine group and sulfonic acid group; 3077.9cm -1 It is the stretching vibration peak of =C-H on the benzene ring; 2960cm -1 for CH 3 Upper stretching vibration peaks; 2922.9 and 2852.2 cm -1 is-CH 2 - C-H stretching vibration; 1905.4 is the out-of-plane bending vibration peak of Ar-H; 1605.5 and 1503.0cm -1 It is the stretching vibration peak of the skeleton on the benzene ring; 1580 and 1450cm -1 It is the third band of substituted benzene ring (characteristic band of conjugated substituted benzene); 1465cm -1 for -CH 2 - deformation vibration; 1299.8 and 1280.5 cm -1 It is the stretching vibration peak of -C-N on the aryl carbon; 1224.3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com