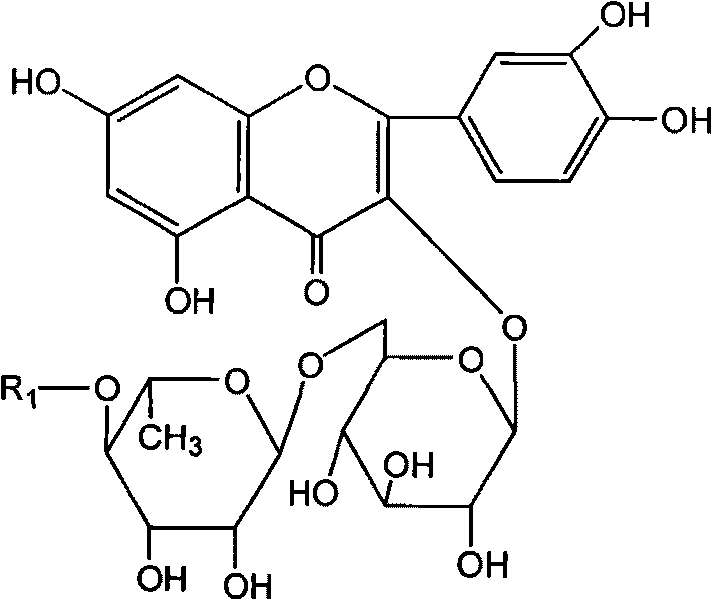
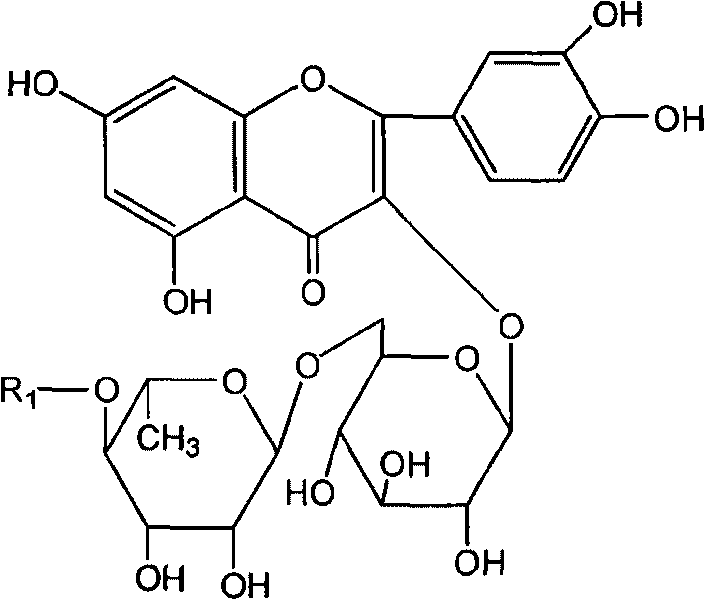
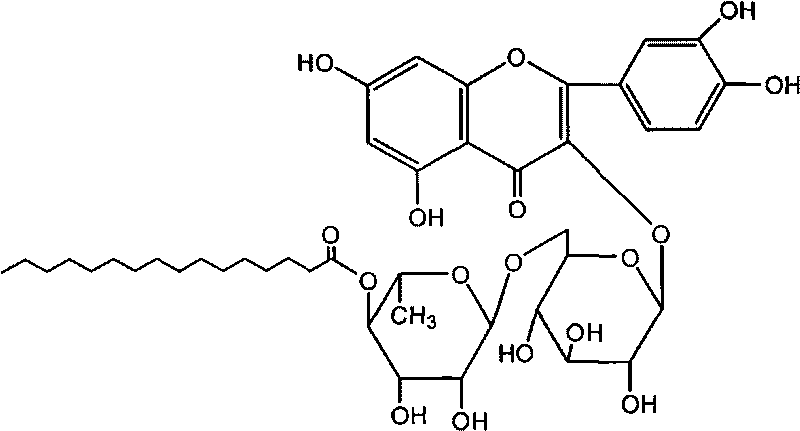
Rutin aliphatic ester derivatives, preparation method and application thereof
A technology of fatty acid esters and derivatives, applied in the field of rutin derivatives, can solve the problems of low bioavailability, insoluble in fat, poor absorption, etc., achieve the effect of simple preparation method, low price, and improved absorption rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The synthesis of embodiment 1 rutin palmitate
[0036]
[0037] Dissolve 0.1mol rutin and 0.5mol palmitic acid in 500ml of tert-butanol, heat, add 2.5g of lipase Novozym435 at 60°C, stir, and react for 10 hours, add 50g of 3A molecular sieve, react for 96 hours, filter to remove molecular sieve and Enzyme, the solution was evaporated to dryness under reduced pressure, and the residue was subjected to silica gel column chromatography, eluent: ethyl acetate-methanol mixture (weight ratio 10:1), the product components were collected, and the solvent was evaporated to dryness to obtain rutin palmitate. Yield 70%. The following spectral data are shown for rutin palmitate.
[0038] Mp: 210-213°C. Electrospray mass spectrometry (ESI-MS) m / z: 871.5 [M+Na].
[0039] 1 H-NMR (C 5 D. 5 N): δ7.53 (2H, m, H-2' and H-6'), 6.79 (1H, d, J=9Hz, H-5'), 6.29 (1H, s, H-8), 6.12 (1H, s, H-6), 5.32 (1H, d, J=7.4Hz, H-1"), 4.65 (1H, t, J=9Hz, H-4'"), 4.48 (1H, s, H-1'"), 3.72-2.65 ...
Embodiment 2
[0041] The synthesis of embodiment 2 rutin myristate
[0042]
[0043] Dissolve 0.1mol of rutin and 0.15mol of myristic acid in 500ml of tert-butanol, heat, add 2.8g of lipase Novozym 435 at 75°C, stir, react for 8 hours, add 40g of 3A molecular sieve, react for 90 hours, filter to remove Molecular sieves and enzymes, the solution was evaporated to dryness under reduced pressure, the residue was subjected to silica gel column chromatography, eluent: ethyl acetate-methanol mixture (weight ratio 12:1), the product components were collected, the solvent was evaporated to dryness, and rutin nutmeg was obtained. Ester, yield 75%. The following spectral data are shown for rutin myristate.
[0044] Mp: 218-220°C. ESI-MS m / z: 843.5 [M+Na].
[0045] 1 H-NMR (C 5 D. 5 N): δ7.52 (2H, m, H-2' and H-6'), 6.78 (1H, d, J=9Hz, H-5'), 6.27 (1H, s, H-8), 6.14 (1H, s, H-6), 5.34 (1H, d, J=7.4Hz, H-1"), 4.67 (1H, t, J=9Hz, H-4'"), 4.49 (1H, s, H-1'"), 3.71-2.63 (9H, m, H2"-H6", H2'"-H5...
Embodiment 3
[0047] Example 3 Synthesis of rutin linolenate
[0048]
[0049] Dissolve 0.2 mol of rutin and 0.6 mol of linolenic acid in 800 ml of tert-butanol, heat, add 4 grams of lipase Novozym435 at 70 ° C, stir, and react for 10 hours, add 100 grams of 3A molecular sieves, react for 100 hours, filter to remove molecular sieves and Enzyme, the solution was evaporated to dryness under reduced pressure, and the residue was subjected to silica gel column chromatography, eluent: ethyl acetate-methanol mixture (weight ratio 15:1), the product components were collected, and the solvent was evaporated to dryness to obtain rutin linolenate. Yield 71%. The following spectral data are shown for rutin linolenate.
[0050] Mp: 202-204°C. ESI-MS m / z: 893.6 [M+Na].
[0051] 1 H-NMR (C 5 D. 5N): δ7.55 (2H, m, H-2' and H-6'), 6.83 (1H, d, J=9Hz, H-5'), 6.27 (1H, s, H-8), 6.11 (1H, s, H-6), 5.34 (1H, d, J=7.4Hz, H-1"), 4.61 (1H, t, J=9Hz, H-4'"), 4.43 (1H, s, H-1'"), 3.71-2.63 (9H, m, H2"-H6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com