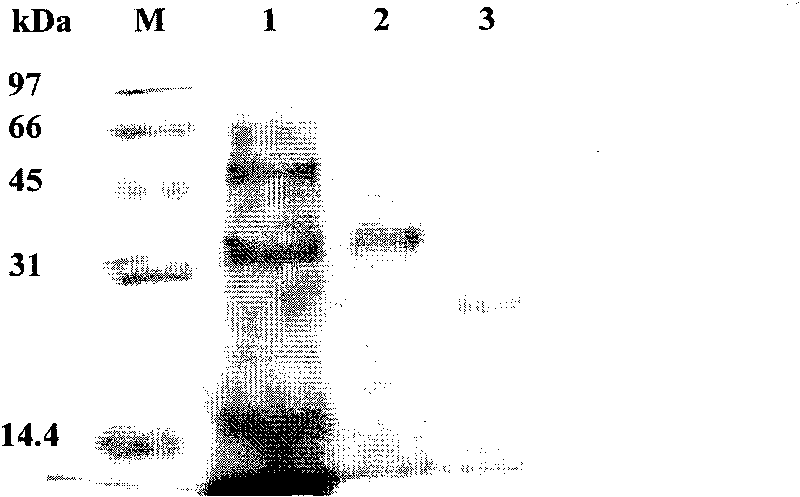N-acylhomoserine lactonas, production method thereof and special recombinant bacterium
A technology of recombinant bacteria and amino acids, applied in the field of genetic engineering, to achieve high-efficiency expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The acquisition of embodiment 1 genetic engineering recombinant bacteria
[0045] 1. Cloning of N-acyl homoserine lactone hydrolase gene
[0046] The genome of Bacillus sp. B546 CGMCC No 3228 was used as a template, using specific primers BT1 (5'-GCGGAATTCATGACAGTAAAGAAAGCTTTATTTCG-3') and BT2 (5'-ATAGCGGCCGCCTATATATACTCTGGGAACAC-3') (the underlined parts are respectively EcoR I and Not I enzyme cutting site), expansion was carried out according to the following conditions: 94°C for 5min; 94°C for 1min, 58°C for 30s, 72°C for 1min, a total of 35 cycles; 72°C for 10min.
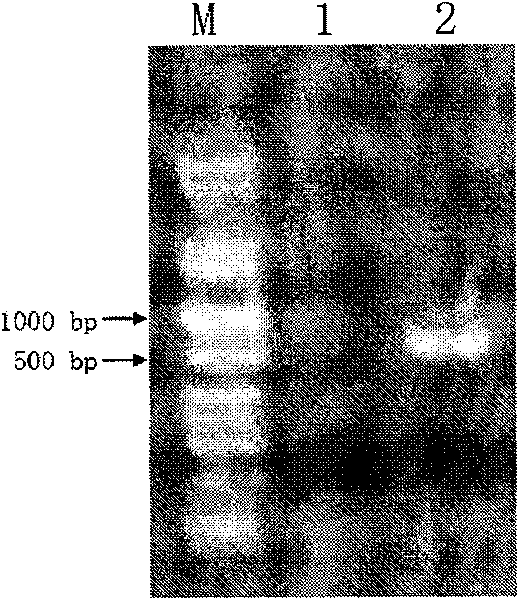
[0047] Amplified fragments of about 750bp (see figure 1 In 2), the pEASY-T3 vector after the recovery of the fragment was transformed into E.coli TOP 10 competent cells, and the positive clones were picked for sequencing. The sequencing results showed that the amplified fragment had the sequence 2 in the sequence table from the 5' The sequence shown from position 1 to position 753 of the 5' end, where...
Embodiment 2
[0060] High expression of embodiment 2AiiA-B546 in P.pastoris AiiA-B546
[0061] 1. Expression and analysis of engineered bacteria at shake flask level
[0062] Re-inoculate the above-mentioned engineering bacteria and empty vector strain control into a 1000mL Erlenmeyer flask containing 200mL of BMGY medium, culture on a shaker at 250-280rpm at 30°C for 48h, centrifuge at 3000×g for 5min, discard the supernatant (clean up as much as possible) , add 200mL of BMMY medium containing 0.5% methanol to the precipitated cells, and re-induce the culture at 25°C, 250-280rpm. The concentration was kept at 0.5% until induction for 72 hours, centrifuged at 12000×g for 5 minutes, and the enzyme activity of the supernatant was measured according to the method provided in Example 1.
[0063] The recombinant Escherichia coli was inoculated in LB liquid medium with Amp (100 μg / ml) and cultivated to OD at 37°C 600=0.6-0.8, add IPTG with a final concentration of 1 mM, and induce culture at 25...
Embodiment 3
[0070] Example 3 Studies on the enzymatic properties of yeast expression AiiA-B546
[0071] Enzyme-catalyzed reaction system: Except for step 4, 3-OXO-C8-HSL was used as the substrate in the remaining steps, and the enzymatic properties of N-acyl homoserine lactonase were determined by the active plate method.
[0072] The enzyme solution used in this example is the AiiA-B546 enzyme solution purified in Example 2.
[0073] 1. The pH and temperature adaptability of AiiA-B546.
[0074] The purified AiiA-B546 was subjected to enzymatic reaction at different pH to determine its optimum pH. The buffer used is 50mM acetic acid-sodium acetate buffer (pH 5.0 and 5.8), 0.2M sodium dihydrogen phosphate-disodium hydrogen phosphate buffer (pH 5.8, 6.0, 7.0 and 8.0), 50mM Tris-HCL buffer (pH 8.0 and 9.0). The pH suitability results of the purified AiiA-B546 in different pH buffer systems at 25°C are as follows: Figure 4 a (take the highest enzyme activity as 100%, and the ratio of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com