Process for preparing low-salt heavy soda ash
A heavy soda ash and preparation technology, applied in the direction of carbonate preparations, etc., can solve the problems of inability to realize large-scale industrial production, non-continuous operation, complicated process, etc., and achieve the effect of simple structure, simple equipment and short process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
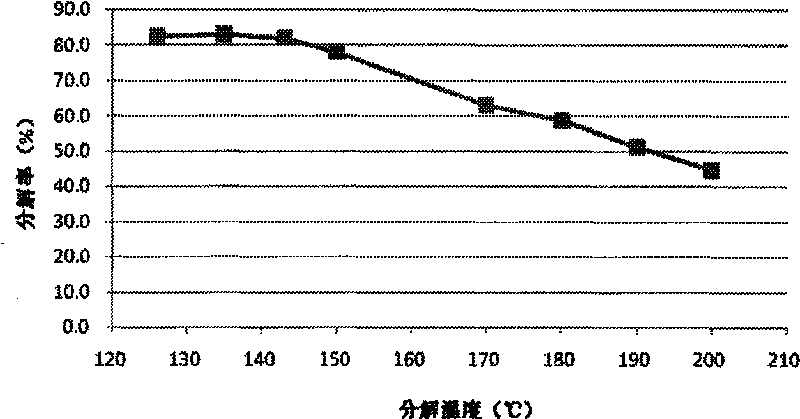
[0030] The preparation method involved in the present invention is based on the inventor's theory and experimental results in this field. attached figure 1 The relationship between the decomposition temperature of precipitated anhydrous sodium carbonate and the minimum decomposition rate of sodium bicarbonate is given. It can be seen that Na 2 CO 3 -NaHCO 3 -H 2 The minimum decomposition rate of sodium bicarbonate required for the precipitation of anhydrous sodium carbonate in the O ternary system is first flat and then decreased with the increase of decomposition temperature. When the decomposition temperature is above 126 ° C, the required minimum decomposition rate is less than 83%. And this decomposition rate is very capable to achieve under the process conditions of pressurized wet decomposition. Applied Na 2 CO 3 -NaHCO 3 -H 2 O ternary system multi-temperature phase diagram, using the characteristics of the system to precipitate anhydrous sodium carbonate cryst...
Embodiment 1
[0038] Na after purification 2 CO 3 53.93g / L, NaHCO 3 The refined natural alkali brine of 81.54g / L and NaCl 4.01g / L (total alkalinity is 105g / L) is continuously added to the sub-tower of wet decomposition and decomposed under normal pressure; Decompose under the pressure of MPa (gauge pressure); the total decomposition time is 0.5h, and the decomposition rate reaches 92.63%. The decomposition liquid is concentrated and crystallized by three-effect evaporation, using 1.0MPa saturated steam as the heat source, and the pressures of I, II, and III effect evaporators are controlled separately At 0.7, 0.5, 0.3MPa, the crystal slurry taken out from the III effect is separated at 130°C to obtain anhydrous sodium carbonate filter cake, which is composed of Na 2 CO 3 97.79%, NaHCO 3 0.21%, NaCl 0.15%, H 2 O 1.85%, after drying, the composition is Na 2 CO 3 99.63%, NaHCO 3 0.21%, NaCl 0.15%, bulk density 0.97g / cm 3 , tightness 1.08g / cm 3 .
Embodiment 2
[0040] Contains NH 4 HCO 3 4.06%, Na 2 CO 3 6.69%, NaHCO 3 67.73%, NaCl 0.64%, H 2 O 20.88% combined alkali intermediate product - wet heavy alkali 17kg, add water 10kg to make slurry to become heavy alkali blending liquid, its composition is NH 4 HCO 3 2.55%, Na 2 CO 3 4.21%, NaHCO 3 42.64%, NaCl0.40%, H 2 O 50.18%, density 1.3g / cm 3 , the total alkalinity is 375g / L. Decompose in the sub-tower of wet decomposition at normal pressure, the main tower of wet decomposition is pressurized at 0.3MPa to decompose, the total decomposition time is 1.0h, and the decomposition rate reaches 88.67%; the decomposition liquid is concentrated and crystallized through three-effect evaporation, and 1.0MPa saturated steam is used as the heat source. The pressures of the II and III effect evaporators were controlled at 0.8, 0.6, and 0.4 MPa respectively, and the crystal slurry taken out from the III effect was separated at 140°C to obtain anhydrous sodium carbonate filter cake, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Bulk density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com