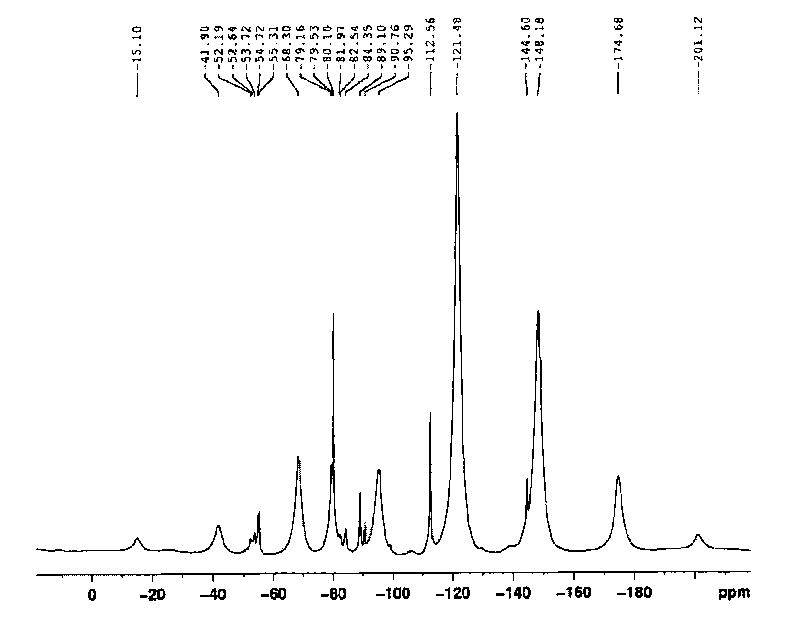
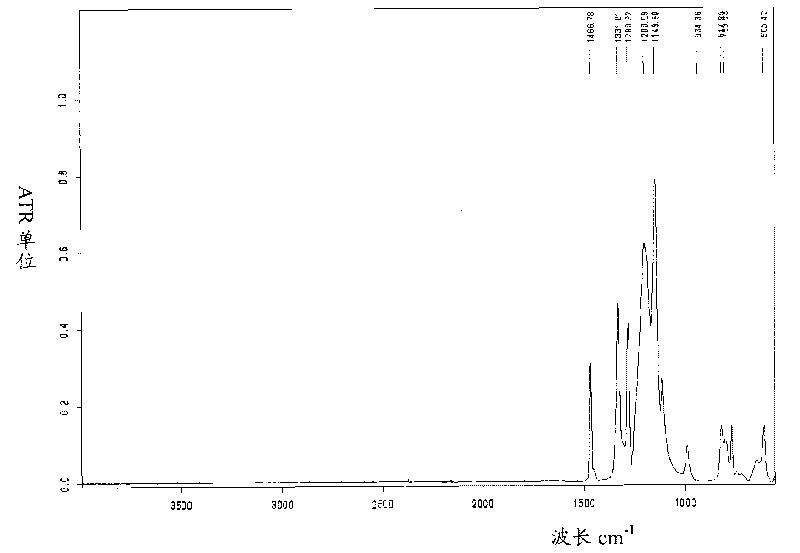
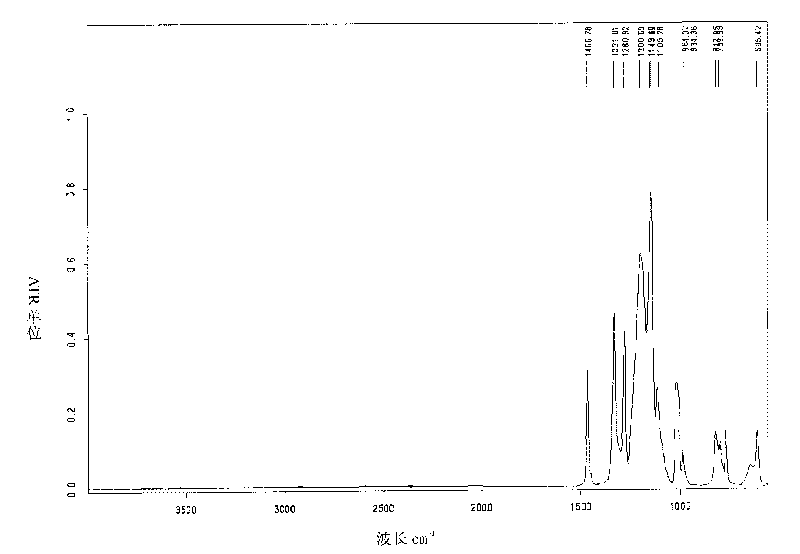
Perfluorinated ion exchange resin with high exchange capacity, preparation method and application thereof
An exchange capacity, perfluororesin technology, applied in chemical instruments and methods, semi-permeable membrane separation, final product manufacturing, etc., can solve problems such as the inability to meet high-temperature use requirements, and achieve good high-temperature mechanical stability and high chemical stability. , the effect of high ion exchange capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Clean the reactor and add 5.0L deionized water, 100g sodium dodecylbenzenesulfonate and 125g nonylphenol polyoxyethylene ether NP-10 emulsifier, start the stirring device, vacuumize and fill with high-purity nitrogen for three times, After testing that the oxygen content in the reaction kettle is below 1ppm, vacuumize, and add 500g of sulfonyl fluoride pendant vinyl ether monomer (1) (F 2 C=CF-O-CF 2 CF 2 -SO 2 F) and 650g sulfonyl fluoride pendant vinyl ether monomer (2) (F 2 C=CF-O-CF 2 CF 2 CF 2 CF 2 -SO 2 F) and 405g bromo pendant vinyl ether monomer (F 2 C=CF-O-CF 2 CF 2 -Br), fill the reaction kettle with tetrafluoroethylene monomer to a pressure of 2.9MPa, raise the temperature to 20°C, and add 2.6g perfluorobutyryl compound (CF 3 CF 2 CF 2 CO-OO-CCF 2 CF 2 CF 3 ) initiates the polymerization reaction, and continuously feeds tetrafluoroethylene (CF 2 = CF 2 ) The monomer keeps the reaction pressure at 2.9 MPa, and adds 0.75 g of initiator to the...
Embodiment 2
[0086] Clean the reactor and add 5.0L of deionized water and 220g of sodium dodecylbenzenesulfonate, start the stirring device, vacuumize and replace with high-purity nitrogen three times, after testing that the oxygen content in the reactor is below 1ppm, vacuumize , add 500g sulfonyl fluoride pendant vinyl ether monomer (1) (F 2 C=CF-O-CF 2 CF 2 -SO 2 F) and 405g sulfonyl fluoride pendant vinyl ether monomer (2) (F 2 C=CF-O-CF 2 CF 2 CF 2 CF 2 -SO 2 F) and 225g bromo pendant vinyl ether monomer (F 2 C=CF-O-CF 2 CF 2 CF 2 After Br), fill the reaction kettle with tetrafluoroethylene monomer to a pressure of 2.9MPa, raise the temperature to 35°C, and add 8.0g perfluoropropoxypropyl peroxide (CF 3 CF 2 CF 2 OCF (CF 3 )CO-OO-CCF(CF 3 )OCF 2 CF 2 CF 3 ) initiates the polymerization reaction, and continuously feeds tetrafluoroethylene (CF 2 = CF 2 ) monomer to keep the reaction pressure at 2.9MPa, add 2.3g of initiator to the system every 25min, stop adding the...
Embodiment 3
[0089] Wash the reactor and add 5.0L deionized water, 120g sodium dodecylbenzenesulfonate and 95g nonylphenol polyoxyethylene ether NP-10 emulsifier, start the stirring device, vacuumize and fill with high-purity nitrogen for three times, After testing that the oxygen content in the reaction kettle is below 1ppm, vacuumize, and add 300g of sulfonyl fluoride pendant vinyl ether monomer (1) (F 2 C=CF-O-CF 2 CF 2 -SO 2 F) and 610g sulfonyl fluoride pendant vinyl ether monomer (2) (F 2 C=CF-O-CF 2 CF 2 CF 2 CF 2 -SO 2 F) and 250g bromo pendant vinyl ether monomer (F 2 C=CF-O-CF 2 CF 2 CF 2 CF 2 After Br), fill the tetrafluoroethylene monomer in the reaction kettle to a pressure of 3.2MPa, heat up to 80°C, add 10% ammonium persulfate aqueous solution 320g to initiate the polymerization reaction with a metering pump, and continuously feed tetrafluoroethylene (CF 2 = CF 2 ) monomer to keep the reaction pressure at 3.2MPa, after reacting for 3h, stop adding tetrafluoroet...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mechanical strength | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com