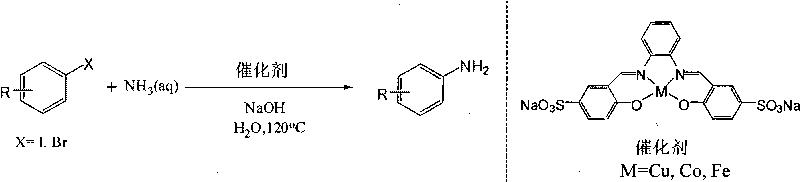
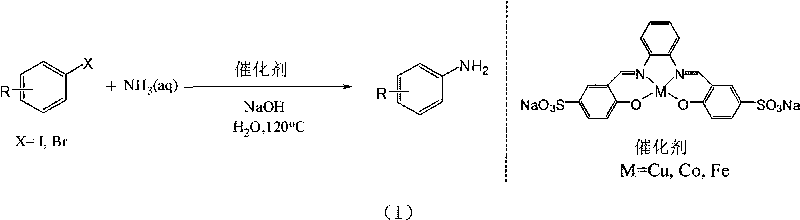
Method for preparing arylamine by catalysis in aqueous phase
A water-phase medium and catalyst technology, which is applied in the preparation of amino compounds, chemical instruments and methods, and the preparation of organic compounds, and can solve problems such as limiting the scope of application of functional groups and harsh reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: iodobenzene prepares aniline
[0034] Add 0.5 mmol (102 mg) of iodobenzene, 0.025 mmol of copper complex, 1 mmol (40 mg) of sodium hydroxide, 1 mL of 25%-28% ammonia water, and 2 mL of water into the reaction vessel. React in an oil bath at 120°C for 12 hours, and cool to room temperature. The product was extracted with ethyl acetate, concentrated under reduced pressure, and the product was purified by column chromatography. A pale yellow oily product was obtained with a yield of 83%. 1 H NMR (CDCl 3 , 400MHz) δ7.15(t, 2H), 6.76(t, 1H), 6.69(d, 2H), 3.52(s, 2H); 13 C NMR (CDCl 3 , 100MHz) δ145.9, 128.5, 117.6, 114.4. MS (EI, m / z): 93 [M + ].
Embodiment 2
[0035] Embodiment 2: p-methyl iodobenzene prepares p-toluidine
[0036] The preparation method is the same as in Example 1, except that 0.5 mmol (108.5 mg) of p-methyliodobenzene is added. A light yellow solid product was obtained with a yield of 86%. 1 H NMR (CDCl 3 , 400MHz) δ6.96(d, 2H), 6.61(d, 2H), 3.42(s, 2H), 2.24(s, 3H). 13 CNMR (CDCl 3 , 100MHz) δ143.8, 129.7, 127.7, 115.3, 20.4. MS (EI, m / z): 107 [M + ].
Embodiment 3
[0037] Embodiment 3: o-methyl iodobenzene prepares o-toluidine
[0038] The preparation method is the same as in Example 1, except that 0.5 mmol (108.5 mg) of o-methyl iodobenzene is added. A light yellow oily product was obtained with a yield of 71%. 1 H NMR (CDCl 3 , 400MHz) δ7.07-7.03(m, 2H), 6.76-6.72(m, 2H), 4.02(s, br, 2H), 2.20(s, 3H); 13 C NMR (CDCl 3 , 100MHz) δ143.0, 129.5, 126.0, 121.7, 118.0, 114.2, 16.3. MS (EI, m / z): 107 [M + ].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com