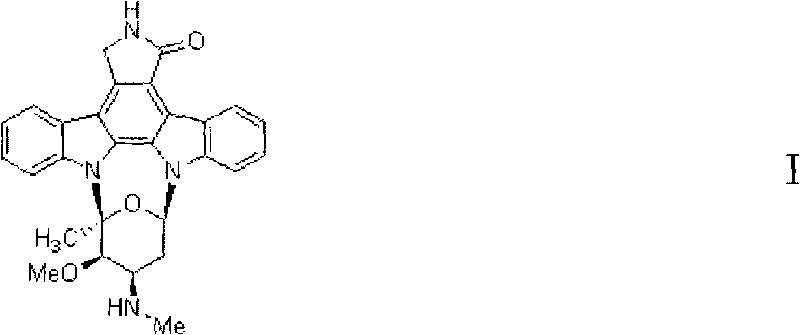
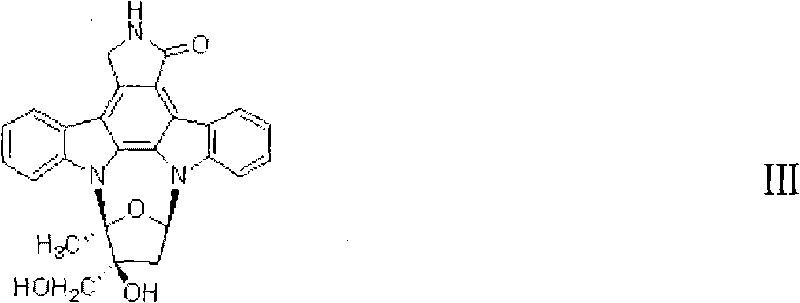
Synthesis method of multi-substituted chiral furanose and intermediate compound
A synthesis method and compound technology, which are applied in the field of synthesis of multi-substituted chiral furanose and intermediate compounds, can solve the problems of hindering research and application, high compound cost, unfavorable industrialization, etc., and achieve reduction of preparation cost, simple operation, Easily adjustable effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0063] (1) Preparation of compound 15
[0064]
[0065] Dissolve 80 g, 0.31 mol of compound 12 in 200 mL of dry dichloromethane, add 45 mL of triethylamine in an ice-water bath, stir for 5 minutes, and slowly add 70.5 g of tert-butyldimethylsilyl chloride dropwise under nitrogen protection, After the addition was complete, the ice-water bath was removed, and the reaction was naturally raised to room temperature for 10 hours. Thin layer chromatography TLC (Thin Layer Chromatography) showed that the raw materials disappeared completely, the reaction solution was poured into 500mL ethyl acetate, the organic phase was washed three times with 200mL water, the organic layers were combined, dried over anhydrous sodium sulfate, filtered and evaporated to dryness under reduced pressure to obtain 92g Compound 15 is a colorless oily liquid with a yield of 90%. The product was directly subjected to the next reaction without further purification.
[0066] (2) Preparation of compound 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com