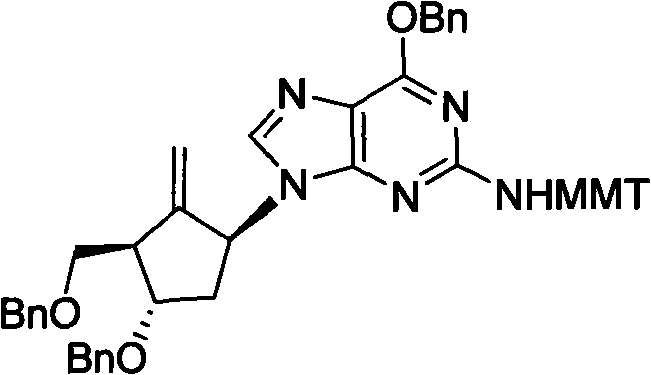
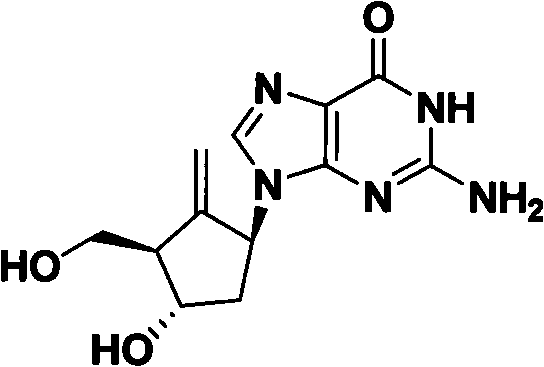
Method for preparing antiviral medicinal entecavir intermediate
An antiviral drug, entecavir technology, applied in organic chemistry and other directions, can solve the problems of extreme sensitivity to air and water vapor, high price, and complicated and complicated homemade process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
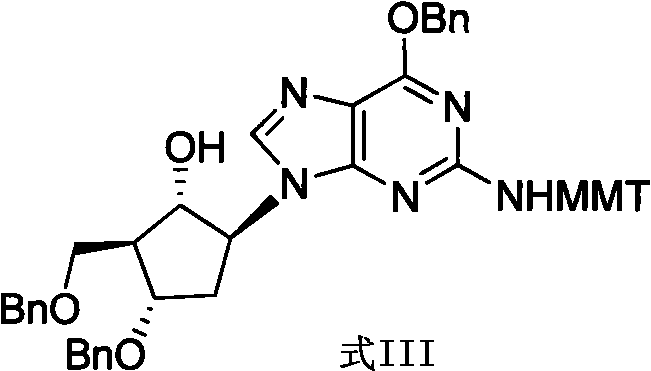
[0027] Example 1 Preparation of (2R, 3S, 5S)-3-benzyloxy-5-[6-benzyloxy-2-[[[(4-methoxyphenyl)diphenylmethyl]amino]- 9H-purin-9-yl]-2-[(benzyloxy)methyl]-cyclopentanone (Formula II)
[0028] Add (1S, 2S, 3S, 5S)-3-benzyloxy-5-[-6-benzyloxy-2-[[(4-methoxyphenyl)benzhydryl] into a 500ml three-necked flask -amino]-9H-purin-9-yl]-2-[(benzyloxy)methyl]-cyclopentanol (formula III) (20g, 24.3mmol) and dichloromethane (300ml), stirred to dissolve, stirred Saturated aqueous sodium bicarbonate solution (60 ml) was added thereto, followed by stirring for 10 minutes. Then iodine (13 g, 51.2 mmol) and TEMPO (2,2,6,6-tetramethylpiperidin-1-oxyl radical) (0.5 g, 3.2 mmol) were added, and the reaction solution was stirred at 20° C. for 5 hours. Cool to 0°C, add 10% sodium sulfite solution (100ml), and stir for 15 minutes. The layers were separated, the aqueous layer was extracted with ethyl acetate (80ml), and the organic phases were combined and washed with saturated potassium bicarbonate...
Embodiment 2
[0029] Example 2 Preparation of (2R, 3S, 5S)-3-benzyloxy-5-[6-benzyloxy-2-[[[(4-methoxyphenyl)diphenylmethyl]amino]- 9H-purin-9-yl]-2-[(benzyloxy)methyl]-cyclopentanone (Formula II)
[0030] The saturated aqueous sodium bicarbonate solution (60ml) in Example 1 was replaced with saturated aqueous sodium carbonate solution (60ml), and other operations were the same as in Example 1, with a yield of 90%.
Embodiment 3
[0031] Example 3 (2R, 3S, 5S)-3-benzyloxy-5-[6-benzyloxy-2-[[[(4-methoxyphenyl)diphenylmethyl]amino]-9H -Purin-9-yl]-2-[(benzyloxy)methyl]-cyclopentanone (Formula II)
[0032] The saturated aqueous sodium bicarbonate solution (60ml) in Example 1 was replaced with a saturated 2M aqueous sodium hydroxide solution (150ml). Other operations were the same as in Example 1, and the yield was 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com