Bisbenzylisoquinoline derivative and preparation and application thereof
A technology of bisbenzylisoquinoline and derivatives, applied in the field of medicine, to achieve good dissolution performance, easy storage and transportation, and easy absorption into the blood circulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
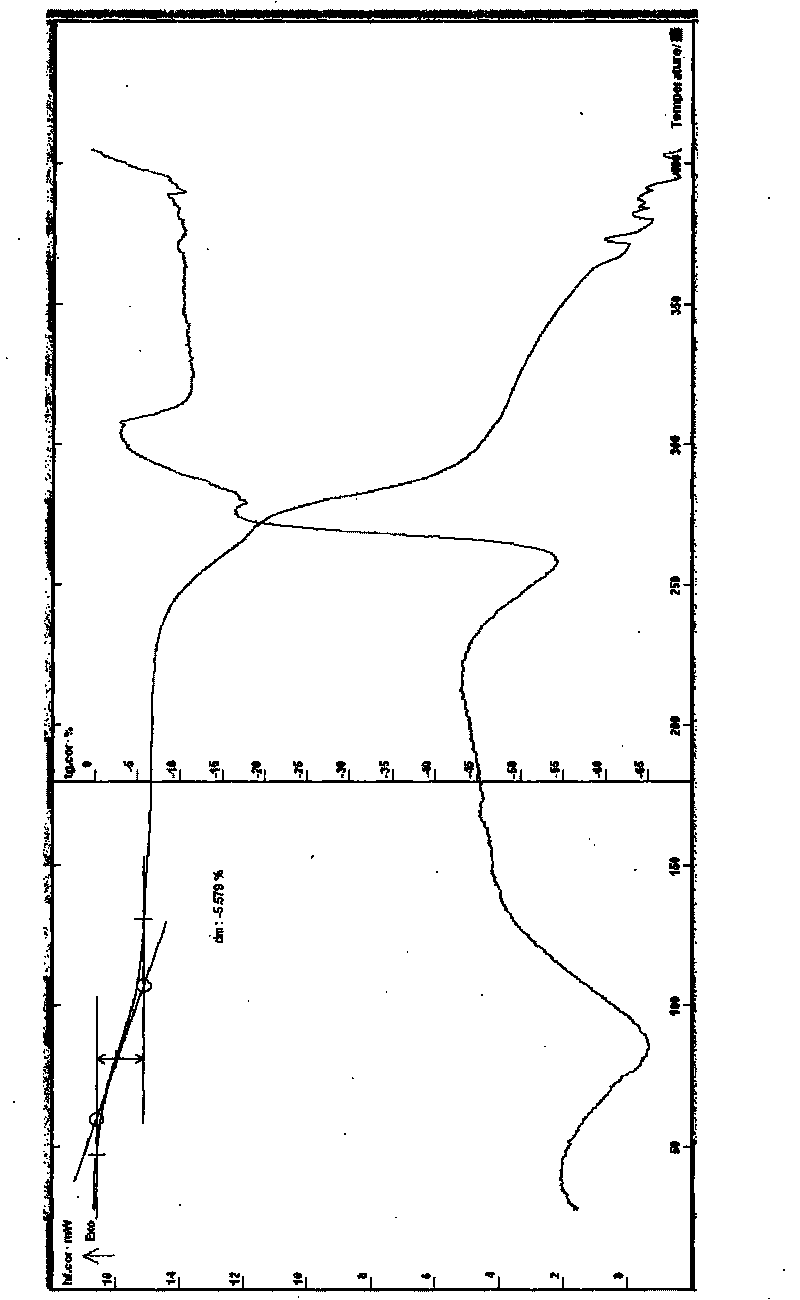
[0035] Example 1 Preparation of dimethyl tetrandrine iodide 3 hydrate Put 3 grams of tetrandrine in a 250ml eggplant-shaped flask, add 100ml of chloroform, heat, stir to dissolve, add 15ml of methyl iodide, control the temperature at 30-80 ℃, stirred for 0.2-3 hours, removed the solvent under reduced pressure, and crystallized the residue with 100ml of ethanol, 5ml of acetone, and 10ml of water, cooled to below 15℃, left to stand until the solid was fully separated out, suction filtered, and dried to obtain Dimethyltetradine iodide crystalline hydrate; recrystallized with 100ml of ethanol, 5ml of acetone, and 10ml of water, cooled to below 15°C, left to stand until the solid was fully separated, filtered with suction, and dried to obtain dimethyltetradine iodide Alkali trihydrate, dry the solid at about 60°C for about 4-5 hours to obtain 2.1g off-white crystals, melting point: 235°C decomposition (uncorrected), [α] D 25 : +179.5°(CH 3 OH, 25 mg (dry note) / ml); take the satur...
Embodiment 2
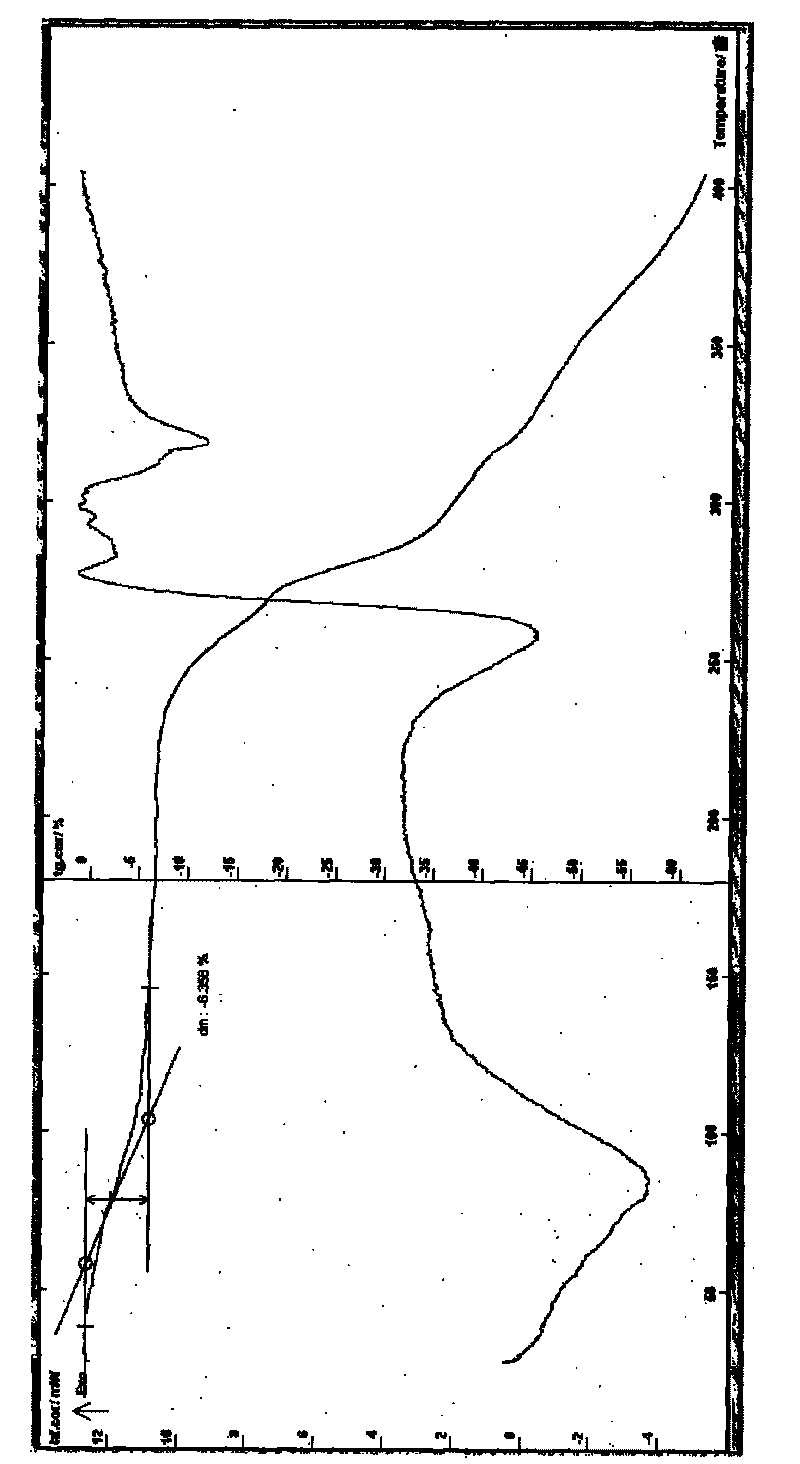
[0036] Example 2 Preparation of dimethyl tetrandrine iodide 3.5 hydrate Put 3 g of tetrandrine in a 500 ml round bottom flask, add 100 ml of dichloromethane, heat, stir to dissolve, add 0.35 g of potassium hydroxide under stirring 38% aqueous solution, add 35ml of methyl iodide dropwise under stirring, control the temperature between 20-70°C, stir and react for 2-5 hours, remove the solvent under reduced pressure, and use methanol 30ml, water 100ml, ethyl acetate 2ml , to carry out crystallization, cool the reactant to below 15°C, place it until the solid is fully separated, filter it with suction, recrystallize the crystal with 30ml of methanol, 1ml of ether, and 100-200ml of water, cool it to below 15°C, place it, and wait for the solid to Fully analyze, filter with suction, and dry the obtained crystals at about 40 ° C for about 4 hours to obtain 1.5 g of dimethyl tetrandrine iodide 3.5 crystalline hydrate, melting point: 234 ° C decomposition (ELECTROTHERMAL MELTING POINT A...
Embodiment 3
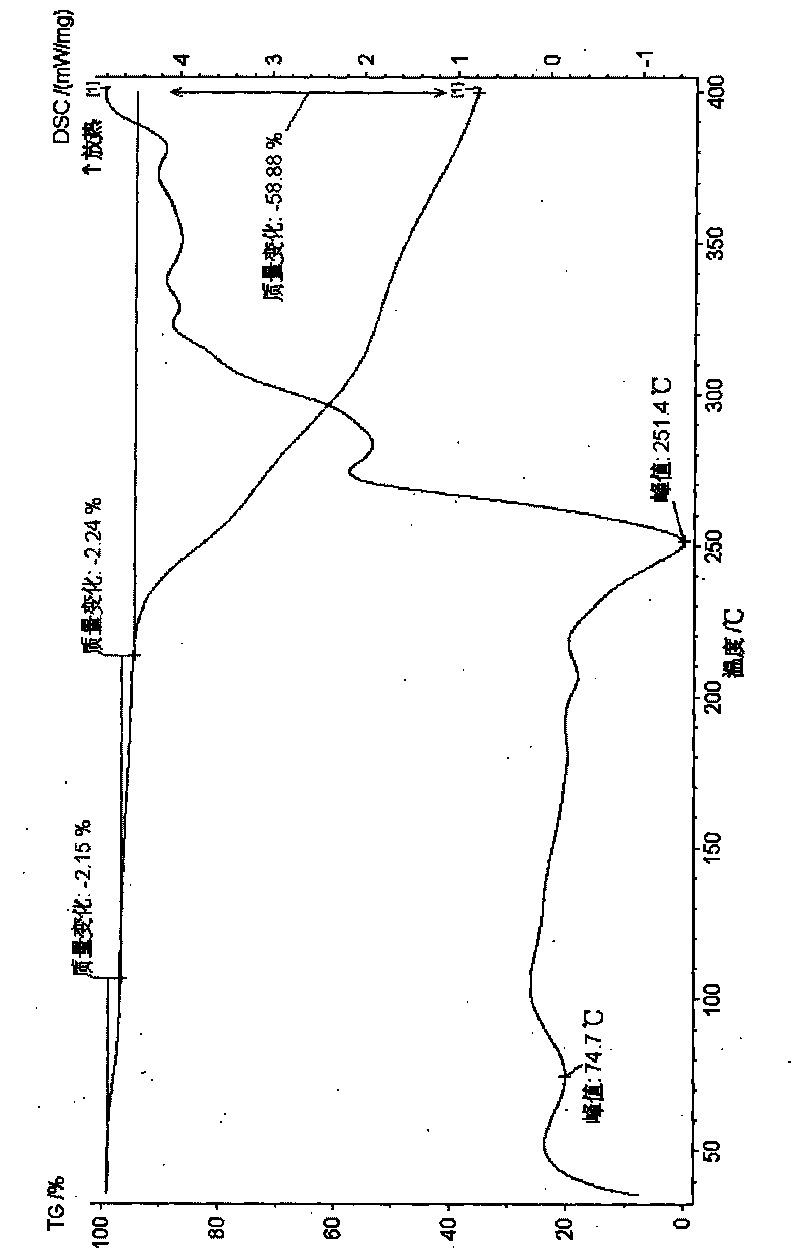
[0037] Example 3 Preparation of dimethyl tetrandrine iodide 1 hydrate Put tetrandrine in a 5g 500ml eggplant-shaped flask, add methanol 100ml, dichloromethane 100ml, heat, stir to dissolve, add iodomethane 25ml, control The temperature is between 30-66°C, stirring and reacting for 0.5-4 hours, the solvent is removed under reduced pressure, the residue is crystallized with methanol, isopropanol and water, cooled to below 15°C, and placed until the solid is fully separated, suction filtered, and dried , to obtain dimethyl tetrandrine iodide crystalline hydrate; use methanol, isopropanol and water to recrystallize, cool to below 15°C, and place it until the solid is fully separated out, and then suction filtered. The obtained solid is in the presence of phosphorus pentoxide for 60 ℃ of vacuum drying, obtain dimethyl tetrandrine iodide 1 hydrate 3.1 grams, Karl Fischer's method measures moisture and is 2.29%, thermal analysis: platform weight loss is about 2.15%, and this and sampl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com