Method for preparing D-(+)-biotin intermediate
A biotin and intermediate technology, applied in chemical instruments and methods, chemical/physical processes, physical/chemical process catalysts, etc., can solve the problem of poor stability and reproducibility of carbonyl reduction coupling reaction, harsh synthesis conditions, mercapto marine products Low toxicity and other problems, to achieve the effect of relatively low toxicity and easy purchase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
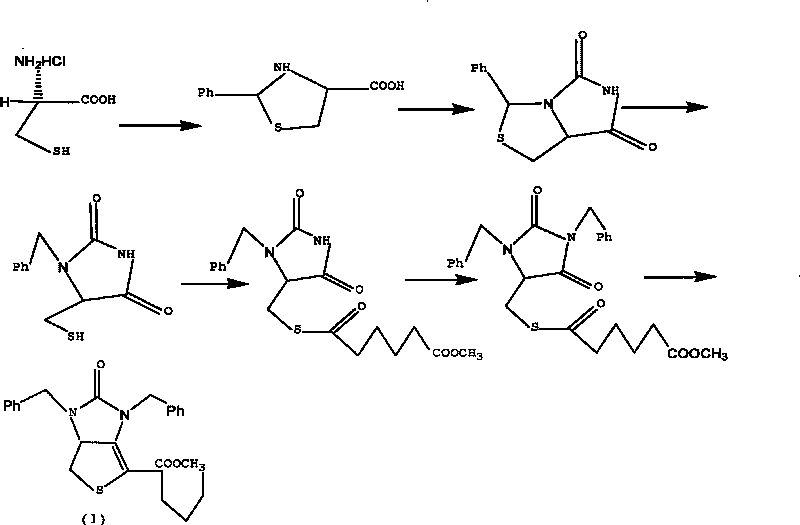
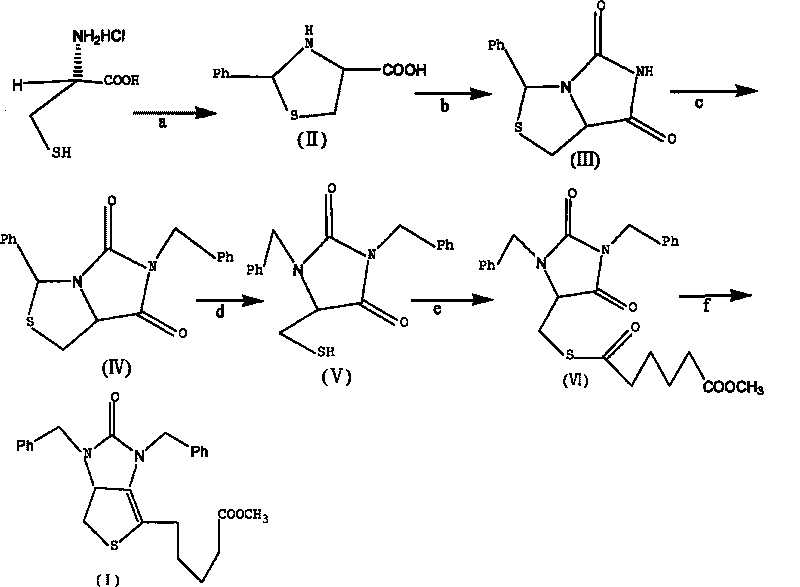
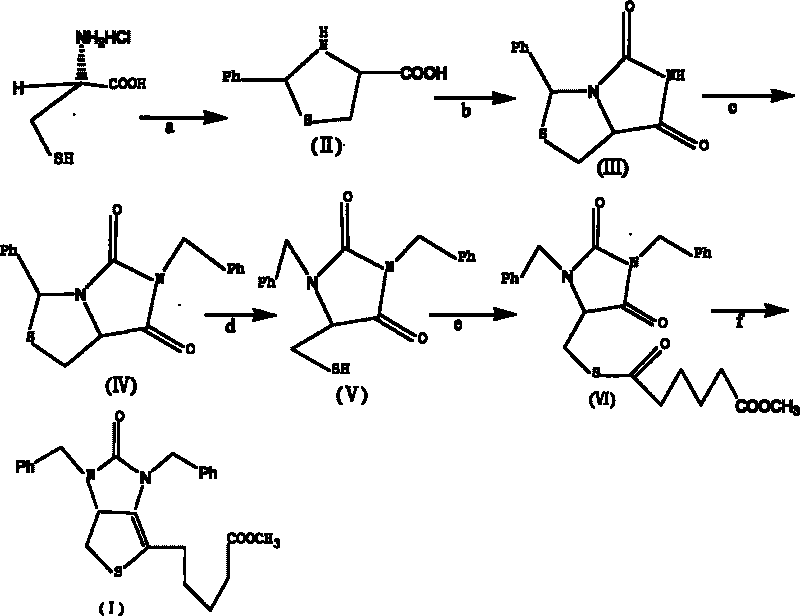
[0039] Example 1 Tetrahydro-(4R)-carboxy-(2s)-phenylthiazole
[0040] Dissolve 50g (0.32mol) of L-cysteine hydrochloride in 500mL of water, add 42g (0.31mol) of sodium acetate trihydrate, and stir for 0.5h. Then add w(C 2 h 5 OH)=250 mL of 95% ethanol, 35 mL of benzaldehyde, and stirred at 20° C. for 3 h. Cool in an ice bath to below 5°C and stir for 2h. After the reaction was completed, it was filtered, and the filter cake was washed twice with ethanol to obtain 70 g of white solid compound II (90% yield).
[0041] 1 HNMR (DMS0), δ: 7.40 (m, 5H), 6.8 (bm, 1H), 5.8 (s, 1H), 4.4-4.0 (dd, 1H), 3.5-3.0 (m, 2H).
[0042] Elemental Analysis C 10 h 11 NO 2 S (209.27) measured value (theoretical value), %: w (C) = 56.87 (57.40), (H) = 5.28 (5.30), w (N) = 6.81 (6.69), w (S) = 15.89 (15.32 ).
Embodiment 2
[0043] Example 2 (7aR)-3-phenyl-1H, 3H-imidazo[1,5-C]thiazole-(6,7aH)-5,7-dione
[0044] Dissolve 30g of compound II and 22g of sodium cyanate obtained in Example 1 in 300mL of glacial acetic acid, heat and reflux in a water bath at 90°C for 12 hours, until the heat of reaction turns into a yellow transparent liquid, spin and concentrate to obtain an oily yellow liquid, which is dissolved in 1000mL In water, a yellow-white precipitate is produced. After filtration, the filter cake was washed with 500 mL of tetrahydrofuran, then saturated brine, dried over anhydrous magnesium sulfate, concentrated by rotation to remove the solvent, and crystallized by adding n-hexane to obtain 27 g of compound III as a yellow solid (yield 80%).
[0045] IR (KBr tablet), v / cm -1 : 1725, 1394; 1 HNMR (CDCl 3 ), δ: 3.20-3.37 (m, 2H), 4.58-4.63 (t, J=7.4Hz, 1H), 7.26-7.46 (m, 5H), 8.67 (br, 1H).
Embodiment 3
[0046] Example 3 (7aR)-3-phenyl-6-benzyl-1H, 3H-imidazo[1,5-C]thiazole-(6H,7aH)5,7-dione
[0047] Dissolve 10.0g of compound III (0.043mol) in 30mL of N,N-dimethylformamide, add 6g of potassium carbonate and 5.5mL of benzyl bromide under stirring, and heat to reflux at 50°C in a water bath for 5h. After the reaction was completed, the insoluble matter was removed by filtration, concentrated by rotation to obtain a viscous liquid, which was dissolved in 45 mL of methanol, stirred at room temperature for 20 min, crystallized at 0° C., and filtered to obtain 12 g of white crystal compound IV (yield 85.7%).
[0048] 1 HNMR (CDCl 3 ), δ: 7.30~7.45 (m, 1OH), 6.41 (s, 1H), 4.67 (s, 2H), 4.52 (t, 1H), 3.30 (dd, 1H), 3.15 (dd, 1H).
[0049] Elemental Analysis C 18 h 16 N 2 o 2 S (324.4) measured value (theoretical value), %: w (C) = 66.41 (66.58), w (H) = 4.91 (4.93), w (N) = 8.71 (8.63), w (S) = 9.95 ( 9.86).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com