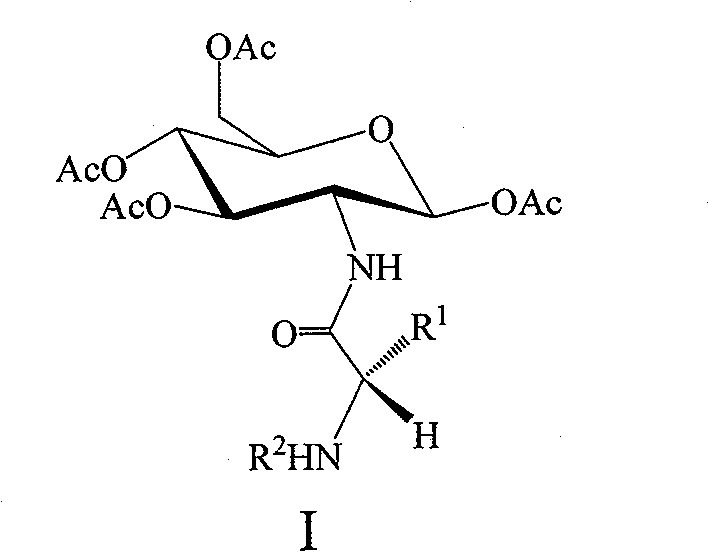
Amino acid modified glucosamine as well as preparation method and application thereof
A glucosamine and amino acid technology, applied in the field of amino acid modified glucosamine, can solve the problems of harsh reaction conditions, long reaction steps, expensive reagents and the like, and achieve the effects of short reaction steps, low cost and convenient post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
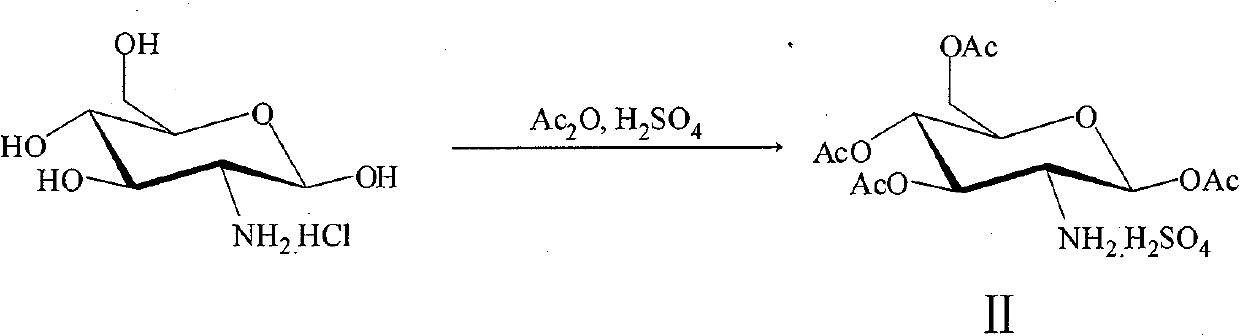
[0028] The preparation of embodiment 1,2-amino-1,3,4,6-tetra-O-acetyl-β-D-glucose sulfate (intermediate II)
[0029] Add acetic anhydride to the round bottom flask, cool in ice-salt bath for 15 minutes, slowly add D-glucosamine hydrochloride, stir rapidly, then slowly add concentrated sulfuric acid dropwise, after the dropwise addition, stir the reaction at room temperature for 24 hours Then stop the reaction, slowly add absolute ethanol dropwise in the ice-salt bath, and a large amount of white solids are precipitated. After the dropwise addition, stir in the ice-salt bath for 30 minutes, filter with suction, wash the filter cake with ethyl acetate until it has no sour smell, and dry it in vacuum at 25°C , to obtain white solid; m.p.168~172℃; 1 H NMR (300MHz, DMSO-d 6 )δ: 6.01(d, 1H, J=3.32Hz, H-1), 5.17(t, 1H, J=9.51Hz, H-3), 4.97(t, 1H, J=9.51Hz, H-4) , 4.37~4.13(m, 3H, H-5, H-6), 3.92(m, 1H, H-2), 2.19, 2.09, 2.02, 1.99(s, 12H, 4×CH 3 ); IR (KBr, cm -1 ): 2986, 2908 (v...
Embodiment 2
[0033] Example 2, N-[N-(9-fluorenylmethoxycarbonyl)alanyl]-1,3,4,6-tetra-O-acetyl-β-D-glucosamine (compound I-1) preparation
[0034]
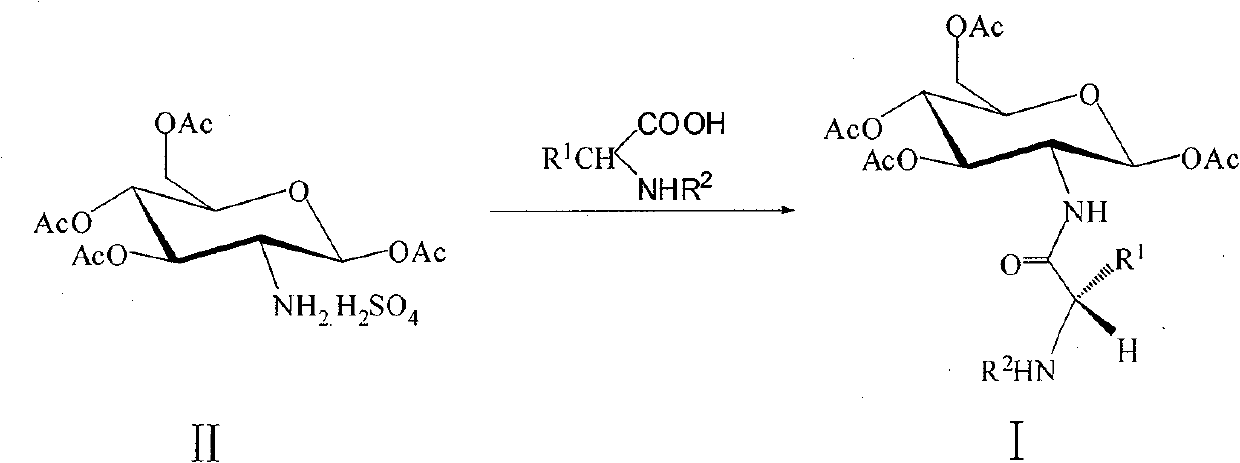
[0035] Dissolve Fmoc-Ala-OH in THF, add DIC, HOBt and DIPEA sequentially under ice bath and stirring, stir for 30 minutes, and set aside; dissolve intermediate II in THF, add triethylamine (Et 3 N), stir evenly, for subsequent use; Above-mentioned two kinds of solutions are mixed, stirring reaction under room temperature, monitor reaction progress with thin-layer chromatography (TLC); Extract with ethyl acetate, collect the aqueous layer and the organic layer respectively, extract the aqueous layer with ethyl acetate again, combine all the organic layers, and successively use a citric acid aqueous solution with a concentration of 0.5mol / L, and sodium bicarbonate with a concentration of 0.5mol / L Wash with aqueous solution and saturated aqueous sodium chloride solution, then dry with anhydrous sodium sulfate, concentrate, and purify by colum...
Embodiment 3
[0045] Example 3, N-[N-(9-fluorenylmethoxycarbonyl) valyl]-1,3,4,6-tetra-O-acetyl-β-D-glucosamine (compound I-2) preparation
[0046]
[0047] Dissolve Fmoc-Val-OH in THF, add DIC, HOBt and DIPEA sequentially under ice bath and stirring, stir for 30 minutes, set aside; dissolve intermediate II in THF, add Et 3 N, stir evenly, set aside; Mix the above two solutions, stir and react at room temperature, monitor the reaction process with TLC method; after the reaction is completed, remove THF by distillation under reduced pressure, add water to the residual liquid, extract with ethyl acetate, and Collect the aqueous layer and the organic layer, and then extract the aqueous layer with ethyl acetate, combine all the organic layers, and successively wash with citric acid aqueous solution with a concentration of 0.5mol / L, sodium bicarbonate aqueous solution with a concentration of 0.5mol / L, and saturated sodium chloride Wash with aqueous solution, dry with anhydrous sodium sulfate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com