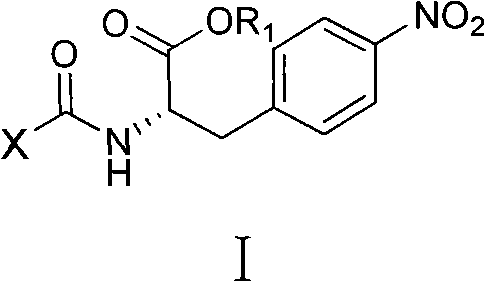
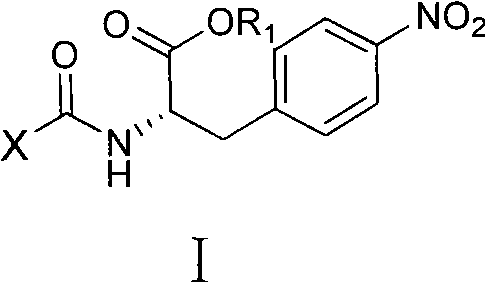
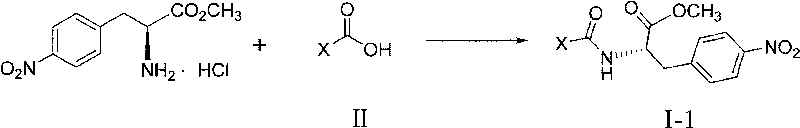
4-nitro-L-phenylalanine dipeptide derivatives as well as preparation method and applications thereof
A technology of nitrophenylalanine dipeptide and derivatives, which is applied in the field of dipeptide derivatives, can solve problems such as large side effects and unsatisfactory treatment effects, and achieve the effects of convenient post-processing, low cost, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The preparation of embodiment 1, L-p-nitrophenylalanine (compound III)
[0037] L-p-nitrophenylalanine can be purchased directly from the market, or can be prepared by yourself. The present invention is prepared by the following improved method: in a round bottom flask, add a mixture of concentrated nitric acid and concentrated sulfuric acid with a volume ratio of 1:1.3, cool in an ice bath to a temperature of 0°C, and slowly add L-phenylalanine in batches , the molar ratio of L-phenylalanine to concentrated nitric acid is 1:1.3. After the addition is completed, the temperature is 0°C and the reaction is stirred for 60 minutes, and then the temperature is raised to room temperature and the reaction is stirred for 60 minutes. After the reaction is completed, a small amount of ice-water mixture is added and stirred. Cool to 0°C, adjust the pH to 6 with concentrated ammonia water in an ice bath, precipitate a large amount of precipitate, refrigerate, filter, wash the filte...
Embodiment 2
[0062] Embodiment 2, the preparation of L-p-nitrophenylalanine methyl ester hydrochloride (compound IV)
[0063] L-p-nitrophenylalanine methyl ester hydrochloride can be purchased directly from the market, or can be prepared by yourself. The present invention is prepared by the following improved method: add anhydrous methanol to a round bottom flask, cool in an ice bath to a temperature of 0°C, slowly add thionyl chloride, after the addition is completed, stir and react at a temperature of 0°C for 20 minutes, and add compound III in batches That is, L-p-nitrophenylalanine, stir until the raw material gradually dissolves into a white turbid liquid, then raise the temperature to 70°C and stir to react, it can be seen that all the raw materials are quickly dissolved and become a yellow clear liquid, and the reaction is monitored by thin layer chromatography (TLC) Process; After the reaction of L-p-nitrophenylalanine is completed, the vacuum rotary distillation is to dryness, the...
Embodiment 3
[0067] Embodiment 3, L-p-nitrophenylalanine dipeptide derivative Fmoc-AA-Phe (p-NO 2 )-OMe Preparation
[0068] General method: Dissolve Fmoc-AA-OH (20mmol) in 10mL of anhydrous THF, add HOBt (24mmol), DIC (24mmol) and DIPEA (20mmol) under ice bath and stirring, after the addition is complete, stir the reaction under ice bath for 30 ~60 minutes, monitor the generation of activated ester with TLC method; Dissolve compound IV, i.e., L-p-nitrophenylalanine methyl ester hydrochloride (20mmol) in 15mL of anhydrous THF, add DIPEA (30mmol), stir, Standby; mix the above two solutions, stir and react at room temperature, and monitor the reaction process by TLC method; after the reaction is completed, remove THF by distillation under reduced pressure, add 150 mL of water to the residual liquid, extract with 150 mL of ethyl acetate, and collect the water layer respectively and the organic layer, and the aqueous layer was extracted with 80mL of ethyl acetate, and all the organic layers w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com