Oligomeric fucosylated glycosaminoglycan and preparation method thereof
A technology of glycosylated glycosylamine and polyfucus, which is applied in the field of medicine, can solve the problems of fucose substitution side chain shedding, high reaction temperature requirements, severe reaction conditions, etc., to reduce the difference between batches and shorten the reaction time course , the effect of improving the reaction speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The peroxide depolymerization of the FG of embodiment 1 metal ion catalysis
[0047] 1.1 Materials:
[0048] FG: Fucosylated glycosaminoglycan derived from Apostichopus japonicus, extracted and prepared according to the literature method (J Biol Chem, 1991, 266(21): 13530-6). Purity 98% (HPGPC, area normalization method), molecular weight (Mw), 69800.
[0049] h 2 o 2 , CH 3 COONa·3H 2 O, NaCl, NaOH, CuCl 2 , FeCl 2 , ZnCl 2 Reagents used: All commercially available analytical reagents.
[0050] 1.2 Method:
[0051] Dissolve 5.0 g of each of the four parts of Apostichopus japonicus FG in 180 ml of water in a round bottom flask, keep warm in a water bath at 45 ° C and continue to stir evenly, add 10 ml of pure water or 20 mmol / L copper chloride (Cu 2+ ) solution, ferrous chloride (Fe 2+ ) solution or zinc chloride (Zn 2+ ) solution, within 2 hours, dropwise add 10% H at a rate of 15ml / h 2 o 2 During the reaction, 1N NaOH solution was used to control the p...
Embodiment 2
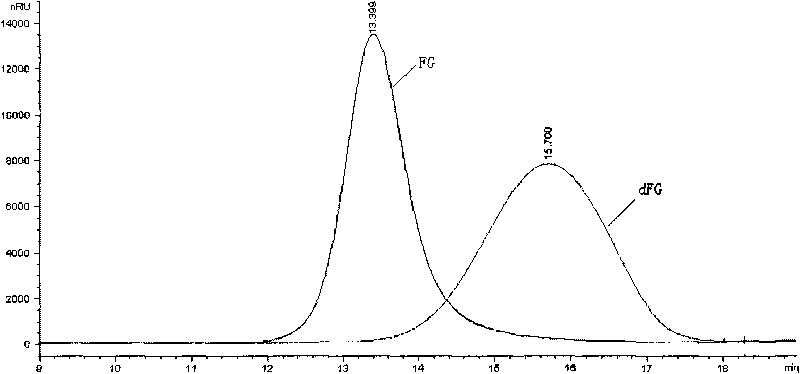
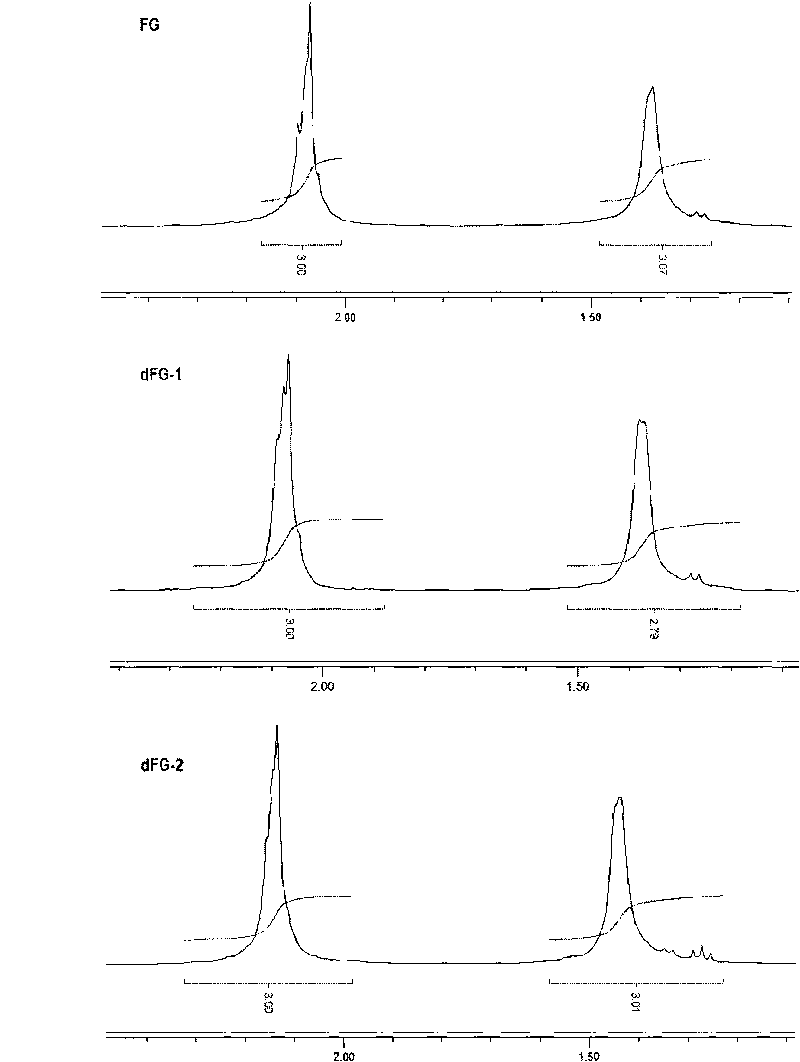
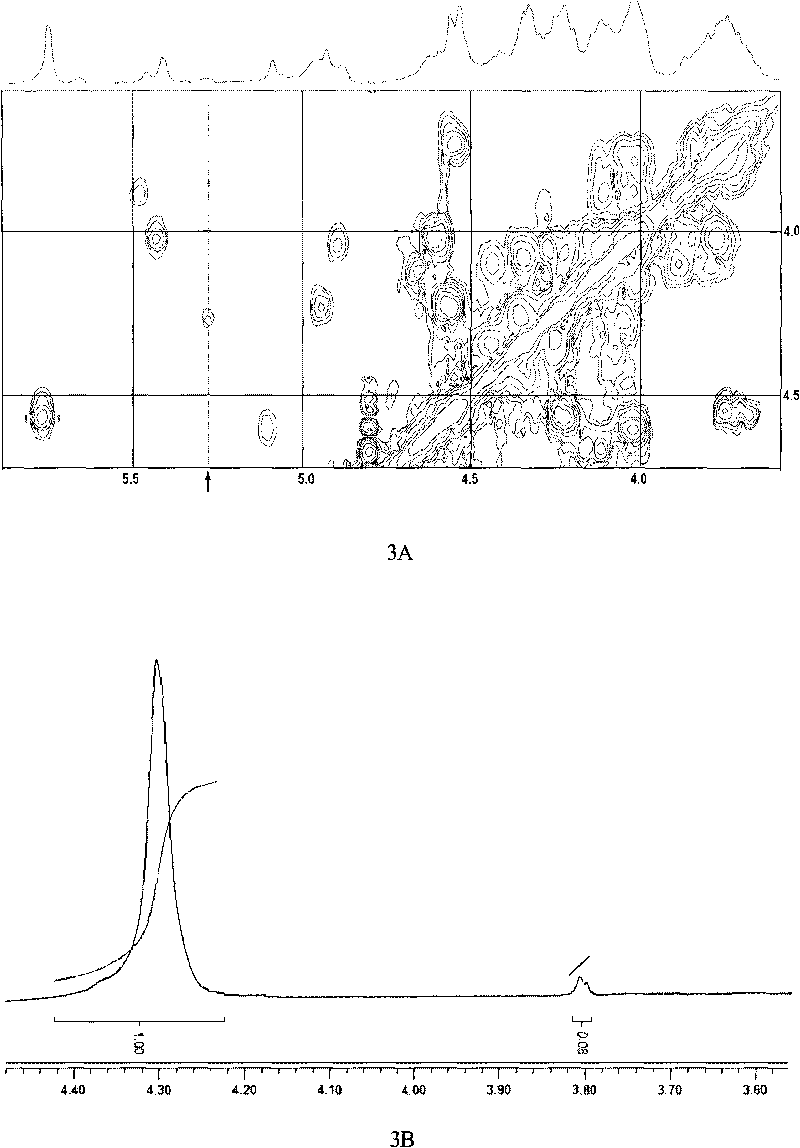
[0057] Embodiment 2 direct peroxide depolymerization method and metal ion catalyzed peroxide depolymerization method gained dFG product NMR spectrum detection
[0058] 2.1 Materials:
[0059] FG: Fucosylated glycosaminoglycan derived from Apostichopus japonicus, extracted and prepared according to the literature method (J Biol Chem, 1991, 266(21): 13530-6). Purity 98% (HPGPC, area normalization method), molecular weight (Mw), 69800.
[0060] h 2 o 2 , CH 3 COONa·3H 2 O, NaCl, NaOH, Cu(CH3COO) 2 ·H 2 Reagents used in O, etc.: All commercially available analytical reagents.
[0061] 2.2 Method:
[0062] Preparation of dFG: Dissolve 5.0g each of two parts of Apostichopus japonicus FG in 180ml of aqueous solution in a round bottom flask, keep warm in a water bath at 70°C and 35°C respectively and keep stirring evenly, then add 10ml of pure water or acetic acid at a concentration of 60mmol / L respectively Copper (Cu 2+ ) solution, and then drop 10% H at a rate of 10ml / h ...
Embodiment 3
[0082] Example 3 The direct peroxide depolymerization method and the dFG prepared by the metal ion catalyzed peroxide depolymerization method Repeatability and controllability comparison
[0083] 3.1 Materials:
[0084] Same as 2.1
[0085] 3.2 Method:
[0086] Depolymerization of FG by direct hydrogen peroxide method: Dissolve 300g of five parts of Apostichopus japonicus FG in 9L of water, keep warm in a water bath at 60°C and continue to stir evenly, then add 15% H2O at a rate of 0.6L / h within 2 hours 2 o 2 , During the reaction process, 1N NaOH solution was used to control the pH value range from 7.2 to 7.8. After continuously stirring and reacting for 6.5 hours under this condition, add 3.0 g of EDTA disodium salt to the reaction solution and mix well, cool with ice water, add 3 times the volume of 95% ethanol to precipitate the polysaccharide, centrifuge to obtain the precipitate, and use 0.6 L of 60% Washed twice with ethanol, then dissolved in 10L water and ultr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com